当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Criegee Intermediates are Enhanced by Hydrogen-Atom Relay Through Molecular Design.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-08-05 , DOI: 10.1002/cphc.202000585 Mei-Tsan Kuo,Kaito Takahashi,Jim Jr-Min Lin
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-08-05 , DOI: 10.1002/cphc.202000585 Mei-Tsan Kuo,Kaito Takahashi,Jim Jr-Min Lin
|
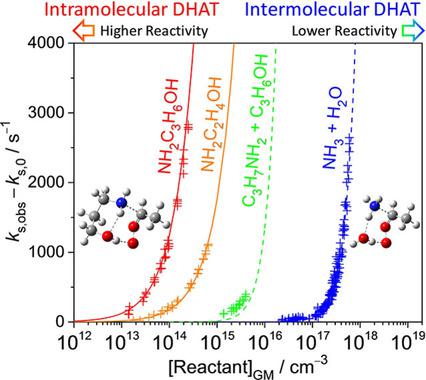
We report a type of highly efficient double hydrogen atom transfer (DHAT) reaction. The reactivities of 3‐aminopropanol and 2‐aminoethanol towards Criegee intermediates (syn‐ and anti‐CH3CHOO) were found to be much higher than those of n‐propanol and propylamine. Quantum chemistry calculation has confirmed that the main mechanism of these very rapid reactions is DHAT, in which the nucleophilic attack of the NH2 group is catalyzed by the OH group which acts as a bridge of HAT. Typical gas‐phase DHAT reactions are termolecular reactions involving two hydrogen bonding molecules; these reactions are typically slow due to the substantial entropy reduction of bringing three molecules together. Putting the reactive and catalytic groups in one molecule circumvents the problem of entropy reduction and allows us to observe the DHAT reactions even at low reactant concentrations. This idea can be applied to improve theoretical predictions for atmospherically relevant DHAT reactions.
中文翻译:

通过分子设计,氢原子中继增强了Criegee中间体的反应。
我们报告了一种高效的双氢原子转移(DHAT)反应。发现3-氨基丙醇和2-氨基乙醇对Criegee中间体(顺式和反式CH 3 CHOO)的反应性比正丙醇和丙胺的反应性高。量子化学计算已证实,这些非常快速的反应的主要机理是DHAT,其中NH 2的亲核攻击该基团被作为HAT的桥的OH基团催化。典型的气相DHAT反应是涉及两个氢键分子的分子反应。由于将三个分子聚集在一起的熵大大降低,这些反应通常很慢。将反应性基团和催化基团放在一个分子中可以避免熵降低的问题,即使在低反应物浓度下,我们也可以观察到DHAT反应。该想法可用于改善与大气有关的DHAT反应的理论预测。
更新日期:2020-09-15
中文翻译:

通过分子设计,氢原子中继增强了Criegee中间体的反应。
我们报告了一种高效的双氢原子转移(DHAT)反应。发现3-氨基丙醇和2-氨基乙醇对Criegee中间体(顺式和反式CH 3 CHOO)的反应性比正丙醇和丙胺的反应性高。量子化学计算已证实,这些非常快速的反应的主要机理是DHAT,其中NH 2的亲核攻击该基团被作为HAT的桥的OH基团催化。典型的气相DHAT反应是涉及两个氢键分子的分子反应。由于将三个分子聚集在一起的熵大大降低,这些反应通常很慢。将反应性基团和催化基团放在一个分子中可以避免熵降低的问题,即使在低反应物浓度下,我们也可以观察到DHAT反应。该想法可用于改善与大气有关的DHAT反应的理论预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号