当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dioxygen Binding to all 3d, 4d, and 5d Transition Metals from Coupled-Cluster Theory.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-08-05 , DOI: 10.1002/cphc.202000529 Klaus A Moltved 1 , Kasper P Kepp 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-08-05 , DOI: 10.1002/cphc.202000529 Klaus A Moltved 1 , Kasper P Kepp 1
Affiliation
|
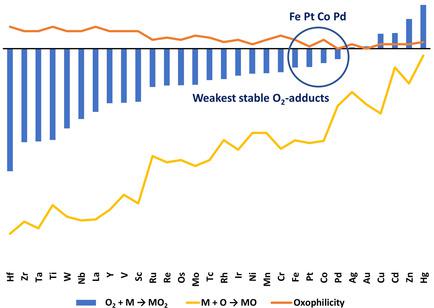
Understanding how transition metals bind and activate dioxygen (O2) is limited by experimental and theoretical uncertainties, making accurate quantum mechanical descriptors of interest. Here we report coupled‐cluster CCSD(T) energies with large basis sets and vibrational and relativistic corrections for 160 3d, 4d, and 5d metal‐O2 systems. We define four reaction energies (120 in total for the 30 metals) that quantify O−O activation and reveal linear relationships between metal‐oxygen and O−O binding energies. The CCSD(T) data can be combined with thermochemical cycles to estimate chemisorption and physisorption energies for each metal from metal oxide embedding energies, in good correlation with atomization enthalpies (R2=0.75). Spin‐geometry variations can break the linearities, of interest to circumventing the Sabatier principle. Pt, Pd, Co, and Fe form a distinct group with the weakest O2 binding. R2 up to 0.84 between surface adsorption energies and our energies for MO2 systems indicate relevance also to real catalytic systems.
中文翻译:

根据耦合簇理论,双氧与所有3d,4d和5d过渡金属的结合。
理解过渡金属如何结合和激活二氧(O 2)受实验和理论不确定性的限制,这使人们对精确的量子力学描述感兴趣。在这里,我们报告了具有160个3d,4d和5d metal-O 2系统的具有大基集的耦合簇CCSD(T)能量以及振动和相对论校正。我们定义了四种反应能(30种金属总共120种),这些能量化O-O活化并揭示金属-氧与O-O结合能之间的线性关系。CCSD(T)数据可与热化学循环结合,以从金属氧化物包埋能估算每种金属的化学吸附能和物理吸附能,并与雾化焓高度相关(R 2= 0.75)。自旋几何形状的变化会破坏线性,这是规避Sabatier原理的兴趣所在。Pt,Pd,Co和Fe形成一个具有最弱O 2结合力的独特基团。表面吸附能和我们的MO 2系统能之间的R 2最高为0.84,这也表明它与真实的催化系统有关。
更新日期:2020-10-02
中文翻译:

根据耦合簇理论,双氧与所有3d,4d和5d过渡金属的结合。
理解过渡金属如何结合和激活二氧(O 2)受实验和理论不确定性的限制,这使人们对精确的量子力学描述感兴趣。在这里,我们报告了具有160个3d,4d和5d metal-O 2系统的具有大基集的耦合簇CCSD(T)能量以及振动和相对论校正。我们定义了四种反应能(30种金属总共120种),这些能量化O-O活化并揭示金属-氧与O-O结合能之间的线性关系。CCSD(T)数据可与热化学循环结合,以从金属氧化物包埋能估算每种金属的化学吸附能和物理吸附能,并与雾化焓高度相关(R 2= 0.75)。自旋几何形状的变化会破坏线性,这是规避Sabatier原理的兴趣所在。Pt,Pd,Co和Fe形成一个具有最弱O 2结合力的独特基团。表面吸附能和我们的MO 2系统能之间的R 2最高为0.84,这也表明它与真实的催化系统有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号