当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CRYSTALLINE ADDUCTS OF UREA WITH MAGNESIUM IODIDE
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129009 K. Kosev , N. Petrova , I. Georgieva , R. Titorenkova , R. Nikolova
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129009 K. Kosev , N. Petrova , I. Georgieva , R. Titorenkova , R. Nikolova
|
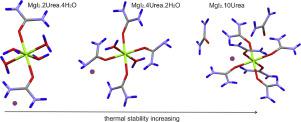
Abstract The crystal structures of newly obtained [Mg(OC(NH2)2)2(H2O)4]I2 (tetraaqua bis (urea-O) magnesium diiodide), [Mg(OC(NH2)2)4(H2O)2]I2 (diaqua tetra(urea-O) magnesium diiodide) and [Mg(OC(NH2)2)6]I2 . 4OC(NH2)2 (hexa(urea-O) magnesium diiodide tetraureate) are reported. It is found that the studied compounds crystalize in one and the same space group P21/c and exhibit structure isotypical with the corresponding chlorides and bromides. The complexes were characterized by experimental IR spectroscopic and DTA-TG analyses, and DFT/B3LYP free energy and vibrational calculations at molecular level. The observed trans-conformation in the crystaline (MgX6)2+units was supported by calculated larger thermodynamic stability of trans- conformers as compared to cis ones. The inclusion of Urea in Mg2+ complexes was preferred over water in agreement with the calculated exothermic exchange reactions. The comparative IR spectroscopic analysis of urea and the three Mg2+ complexes studied revealed that ν(C = O)U band shift could be an indication of Mg - OU bond formation and its relative strength. The thermal analyses and calculated exchange reaction energies predicted larger stability of the Mg2+ complexes with an increase of the number of the urea (respectively a decrease of water's number in the first coordinating shell) due to formation of specific network of hydrogen bonds.
中文翻译:

尿素与碘化镁的结晶加合物
摘要 新获得的 [Mg(OC(NH2)2)2(H2O)4]I2 (tetraaqua bis (urea-O)二碘化镁), [Mg(OC(NH2)2)4(H2O)2] 的晶体结构I2 (diaqua tetra(urea-O)二碘化镁)和[Mg(OC(NH2)2)6]I2。报道了 4OC(NH2)2(六(脲-O)二碘化镁四脲)。发现所研究的化合物在一个相同的空间群 P21/c 中结晶,并表现出与相应氯化物和溴化物同型的结构。通过实验红外光谱和 DTA-TG 分析以及分子水平的 DFT/B3LYP 自由能和振动计算来表征复合物。在晶体 (MgX6)2+ 单元中观察到的反式构象得到了计算出的反式构象体与顺式构象相比更大的热力学稳定性的支持。与计算出的放热交换反应一致,在 Mg2+ 复合物中加入尿素优于水。尿素和所研究的三种 Mg2+ 配合物的比较红外光谱分析表明,ν(C = O)U 带位移可能是 Mg - OU 键形成及其相对强度的指示。热分析和计算的交换反应能预测,由于形成特定的氢键网络,随着尿素数量的增加(分别是第一配位壳中水的数量减少),Mg2+ 络合物的稳定性更大。尿素和所研究的三种 Mg2+ 配合物的比较红外光谱分析表明,ν(C = O)U 带位移可能是 Mg - OU 键形成及其相对强度的指示。热分析和计算的交换反应能预测,由于形成特定的氢键网络,随着尿素数量的增加(分别是第一配位壳中水的数量减少),Mg2+ 络合物的稳定性更大。尿素和所研究的三种 Mg2+ 配合物的比较红外光谱分析表明,ν(C = O)U 带位移可能是 Mg - OU 键形成及其相对强度的指示。热分析和计算的交换反应能预测,由于形成特定的氢键网络,随着尿素数量的增加(分别是第一配位壳中水的数量减少),Mg2+ 络合物的稳定性更大。
更新日期:2021-01-01
中文翻译:

尿素与碘化镁的结晶加合物
摘要 新获得的 [Mg(OC(NH2)2)2(H2O)4]I2 (tetraaqua bis (urea-O)二碘化镁), [Mg(OC(NH2)2)4(H2O)2] 的晶体结构I2 (diaqua tetra(urea-O)二碘化镁)和[Mg(OC(NH2)2)6]I2。报道了 4OC(NH2)2(六(脲-O)二碘化镁四脲)。发现所研究的化合物在一个相同的空间群 P21/c 中结晶,并表现出与相应氯化物和溴化物同型的结构。通过实验红外光谱和 DTA-TG 分析以及分子水平的 DFT/B3LYP 自由能和振动计算来表征复合物。在晶体 (MgX6)2+ 单元中观察到的反式构象得到了计算出的反式构象体与顺式构象相比更大的热力学稳定性的支持。与计算出的放热交换反应一致,在 Mg2+ 复合物中加入尿素优于水。尿素和所研究的三种 Mg2+ 配合物的比较红外光谱分析表明,ν(C = O)U 带位移可能是 Mg - OU 键形成及其相对强度的指示。热分析和计算的交换反应能预测,由于形成特定的氢键网络,随着尿素数量的增加(分别是第一配位壳中水的数量减少),Mg2+ 络合物的稳定性更大。尿素和所研究的三种 Mg2+ 配合物的比较红外光谱分析表明,ν(C = O)U 带位移可能是 Mg - OU 键形成及其相对强度的指示。热分析和计算的交换反应能预测,由于形成特定的氢键网络,随着尿素数量的增加(分别是第一配位壳中水的数量减少),Mg2+ 络合物的稳定性更大。尿素和所研究的三种 Mg2+ 配合物的比较红外光谱分析表明,ν(C = O)U 带位移可能是 Mg - OU 键形成及其相对强度的指示。热分析和计算的交换反应能预测,由于形成特定的氢键网络,随着尿素数量的增加(分别是第一配位壳中水的数量减少),Mg2+ 络合物的稳定性更大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号