当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Biological Assessment and Molecular Docking Studies of Some New 2-Thioxo-2,3-dihydropyrimidin-4(1H)-ones as potential Anticancer and Antibacterial agents
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.molstruc.2020.129014 Abd-Allah Sh El-Etrawy , Farag F. Sherbiny
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.molstruc.2020.129014 Abd-Allah Sh El-Etrawy , Farag F. Sherbiny
|
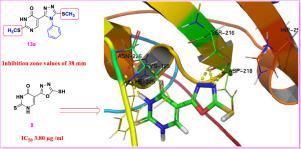
Abstract In this study, a series of thiouracil derivatives were designed and synthesized based on conventional approach and pharmacophoric features essential for TS inhibitors. The chemical structures of all synthesized compounds were elucidated by various techniques ranging from micro-elemental analyses to spectral analyses. The new thiouracil derivatives were evaluated for their anticancer and antibacterial activities. All the new synthesized compounds were evaluated in vitro against MCF-7 cell line. The anticancer results displayed that compounds 8, 11, 13a, and 12, exhibit the highly significant effect against breast cancer cell line with IC50 values of 3.80, 4.00, 4.50, and 4.70 µg/ml, respectively compared with doxorubicin. Furthermore, molecular docking studies were performed to suggest possible mechanism of action of the designed compounds and explain the anti-breast cancer results with prospective target, thymidylate synthase (PDB:1JU6). On the other hand, the antibacterial activity of the new compounds was screened against three significant representative strains including Escherichia coli, and Pseudomonas aeruginosa as gram negative bacterium and Staphylococcus aureus as gram positive bacterium using agar well diffusion method. The antibacterial activity results revealed that most of the tested compounds exhibited significant antibacterial activity. In particularly, compounds 13a, and 13b were found to be the most potent antibacterial agent with inhibition zone values of 38 and 35 mm at the concentration of 50 µg/ml against Escherichia coli, and inhibition zone values of 25 and 23 mm at the concentration of 50 µg/ml against Staphylococcus aureus. However, all tested strains showed resistance to synthesized compounds except, compound 7 which exhibited significant activity only against Pseudomonas aeruginosa with inhibition zone values of 22 mm at the concentration of 50 µg/ml. Further, molecular docking investigation was carried out to gain insight into the binding mode of the most promising compounds using crystal structure of S. aureus DNA gyrase complex with ciprofloxacin (PDB ID: 2XCT).
中文翻译:

一些新型 2-Thioxo-2,3-dihydropyrimidin-4(1H)-ones 作为潜在抗癌和抗菌剂的设计、合成、生物学评估和分子对接研究
摘要 在本研究中,基于 TS 抑制剂所必需的常规方法和药效特征,设计并合成了一系列硫尿嘧啶衍生物。所有合成化合物的化学结构都通过从微量元素分析到光谱分析的各种技术来阐明。评估了新的硫尿嘧啶衍生物的抗癌和抗菌活性。所有新合成的化合物都在体外针对 MCF-7 细胞系进行了评估。抗癌结果显示,化合物8、11、13a和12对乳腺癌细胞系表现出非常显着的作用,与阿霉素相比,IC50值分别为3.80、4.00、4.50和4.70 μg/ml。此外,进行分子对接研究以提出设计化合物的可能作用机制,并用前瞻性靶标胸苷酸合酶 (PDB:1JU6) 解释抗乳腺癌结果。另一方面,使用琼脂孔扩散法筛选了新化合物对三种重要的代表性菌株的抗菌活性,包括作为革兰氏阴性菌的大肠杆菌和作为革兰氏阴性菌的铜绿假单胞菌和作为革兰氏阳性菌的金黄色葡萄球菌。抗菌活性结果表明,大多数测试化合物表现出显着的抗菌活性。特别是,发现化合物 13a 和 13b 是最有效的抗菌剂,浓度为 50 µg/ml 时对大肠杆菌的抑菌圈值为 38 和 35 mm,浓度为 50 µg/ml 时,对金黄色葡萄球菌的抑菌圈值为 25 和 23 mm。然而,所有测试菌株均显示对合成化合物的抗性,但化合物 7 仅对铜绿假单胞菌表现出显着活性,在 50 µg/ml 浓度下抑制区值为 22 mm。此外,使用金黄色葡萄球菌 DNA 促旋酶复合物与环丙沙星(PDB ID:2XCT)的晶体结构,进行了分子对接研究,以深入了解最有希望的化合物的结合模式。化合物 7 仅对铜绿假单胞菌表现出显着活性,在 50 µg/ml 的浓度下抑制区值为 22 mm。此外,使用金黄色葡萄球菌 DNA 促旋酶复合物与环丙沙星(PDB ID:2XCT)的晶体结构,进行了分子对接研究,以深入了解最有希望的化合物的结合模式。化合物 7 仅对铜绿假单胞菌表现出显着活性,在 50 µg/ml 的浓度下抑制区值为 22 mm。此外,使用金黄色葡萄球菌 DNA 促旋酶复合物与环丙沙星(PDB ID:2XCT)的晶体结构,进行了分子对接研究,以深入了解最有希望的化合物的结合模式。
更新日期:2021-02-01
中文翻译:

一些新型 2-Thioxo-2,3-dihydropyrimidin-4(1H)-ones 作为潜在抗癌和抗菌剂的设计、合成、生物学评估和分子对接研究
摘要 在本研究中,基于 TS 抑制剂所必需的常规方法和药效特征,设计并合成了一系列硫尿嘧啶衍生物。所有合成化合物的化学结构都通过从微量元素分析到光谱分析的各种技术来阐明。评估了新的硫尿嘧啶衍生物的抗癌和抗菌活性。所有新合成的化合物都在体外针对 MCF-7 细胞系进行了评估。抗癌结果显示,化合物8、11、13a和12对乳腺癌细胞系表现出非常显着的作用,与阿霉素相比,IC50值分别为3.80、4.00、4.50和4.70 μg/ml。此外,进行分子对接研究以提出设计化合物的可能作用机制,并用前瞻性靶标胸苷酸合酶 (PDB:1JU6) 解释抗乳腺癌结果。另一方面,使用琼脂孔扩散法筛选了新化合物对三种重要的代表性菌株的抗菌活性,包括作为革兰氏阴性菌的大肠杆菌和作为革兰氏阴性菌的铜绿假单胞菌和作为革兰氏阳性菌的金黄色葡萄球菌。抗菌活性结果表明,大多数测试化合物表现出显着的抗菌活性。特别是,发现化合物 13a 和 13b 是最有效的抗菌剂,浓度为 50 µg/ml 时对大肠杆菌的抑菌圈值为 38 和 35 mm,浓度为 50 µg/ml 时,对金黄色葡萄球菌的抑菌圈值为 25 和 23 mm。然而,所有测试菌株均显示对合成化合物的抗性,但化合物 7 仅对铜绿假单胞菌表现出显着活性,在 50 µg/ml 浓度下抑制区值为 22 mm。此外,使用金黄色葡萄球菌 DNA 促旋酶复合物与环丙沙星(PDB ID:2XCT)的晶体结构,进行了分子对接研究,以深入了解最有希望的化合物的结合模式。化合物 7 仅对铜绿假单胞菌表现出显着活性,在 50 µg/ml 的浓度下抑制区值为 22 mm。此外,使用金黄色葡萄球菌 DNA 促旋酶复合物与环丙沙星(PDB ID:2XCT)的晶体结构,进行了分子对接研究,以深入了解最有希望的化合物的结合模式。化合物 7 仅对铜绿假单胞菌表现出显着活性,在 50 µg/ml 的浓度下抑制区值为 22 mm。此外,使用金黄色葡萄球菌 DNA 促旋酶复合物与环丙沙星(PDB ID:2XCT)的晶体结构,进行了分子对接研究,以深入了解最有希望的化合物的结合模式。


































 京公网安备 11010802027423号
京公网安备 11010802027423号