Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-08-05 , DOI: 10.1016/j.jmgm.2020.107708 Rajalakshmi Kumar 1 , Manikandan Jayaraman 2 , Krishna Ramadas 2 , Adithan Chandrasekaran 1
|
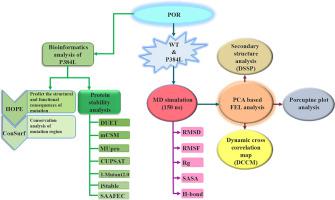
Cytochrome P450 oxidoreductase (POR) is a steroidogenic and drug-metabolizing enzyme. It helps in the NADPH dependent transfer of electrons to cytochrome P450 (CYP) enzymes for their biological activity. In this study, we employed integrative computational approaches to decipher the impact of proline to leucine missense mutation at position 384 (P384L) in the connecting/hinge domain region which is essential for the catalytic activity of POR. Analysis of protein stability using DUET, MUpro, CUPSAT, I-Mutant2.0, iStable and SAAFEC servers predicted that mutation might alter the structural stability of POR. The significant conformational changes induced by the mutation to the POR structure were analyzed by long-range molecular dynamics simulation. The results revealed that missense mutation decreased the conformational stability of POR as compared to wild type (WT). The PCA based FEL analysis described the mutant-specific conformational alterations and dominant motions essential for the biological activity of POR. The LIGPLOT interaction analysis showed the different binding architecture of FMN, FAD, and NADPH as a result of mutation. The increased number of hydrogen bonds in the FEL conformation of WT proved the strong binding of cofactors in the binding pocket as compared to the mutant. The porcupine plot analysis associated with cross-correlation analysis depicted the high-intensity flexible motion exhibited by functionally important FAD and NADPH binding domain regions. The computational findings unravel the impact of mutation at the structural level, which could be helpful in understanding the molecular mechanism of drug metabolism.
中文翻译:

深入了解错义突变对细胞色素P450氧化还原酶的影响的结构和功能分析。
细胞色素P450氧化还原酶(POR)是类固醇生成和药物代谢酶。它有助于将NADPH依赖的电子转移至细胞色素P450(CYP)酶,以实现其生物学活性。在这项研究中,我们采用综合计算方法来分析脯氨酸对连接/铰链域区域中384位(P384L)亮氨酸错义突变的影响,这对POR的催化活性至关重要。使用DUET,MUpro,CUPSAT,I-Mutant2.0,iStable和SAAFEC服务器进行的蛋白质稳定性分析预测,突变可能会改变POR的结构稳定性。通过远程分子动力学模拟分析了突变导致的POR结构的显着构象变化。结果表明,与野生型(WT)相比,错义突变降低了POR的构象稳定性。基于PCA的FEL分析描述了POR生物学活性必不可少的突变体特异性构象变化和显性运动。LIGPLOT相互作用分析表明,由于突变,FMN,FAD和NADPH的结合结构不同。WT的FEL构象中增加的氢键数量证明了与突变体相比,辅因子在结合袋中的牢固结合。与互相关分析相关的豪猪图分析描绘了功能上重要的FAD和NADPH结合域区域所表现出的高强度灵活运动。计算结果揭示了突变在结构水平上的影响,



























 京公网安备 11010802027423号
京公网安备 11010802027423号