Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 4.6 ) Pub Date : 2020-08-05 , DOI: 10.1016/j.bbamcr.2020.118815 Rosella Scrima 1 , Olga Cela 1 , Francesca Agriesti 2 , Claudia Piccoli 1 , Tiziana Tataranni 2 , Consiglia Pacelli 1 , Gianluigi Mazzoccoli 3 , Nazzareno Capitanio 1
|
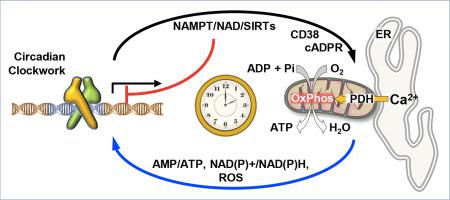
Regulation of metabolism is emerging as a major output of circadian clock circuitry in mammals. Accordingly, mitochondrial oxidative metabolism undergoes both in vivo and in vitro daily oscillatory activities. In a previous study we showed that both glycolysis and mitochondrial oxygen consumption display a similar time-resolved rhythmic activity in synchronized HepG2 cell cultures, which translates in overall bioenergetic changes as here documented by measurement of the ATP level. Treatment of synchronized cells with specific metabolic inhibitors unveiled pyruvate as a major source of reducing equivalents to the respiratory chain with its oxidation driven by the rhythmic (de)phosphorylation of pyruvate dehydrogenase. Further investigation enabled to causally link the autonomous cadenced mitochondrial respiration to a synchronous increase of the mitochondrial Ca2+. The rhythmic change of the mitochondrial respiration was dampened by inhibitors of the mitochondrial Ca2+ uniporter as well as of the ryanodine receptor Ca2+ channel or the ADPR cyclase, indicating that the mitochondrial Ca2+ influx originated from the ER store, likely at contact sites with the mitochondrial compartment. Notably, blockage of the mitochondrial Ca2+ influx resulted in deregulation of the expression of canonical clock genes such as BMALl1, CLOCK, NR1D1. All together our findings unveil a hitherto unexplored function of Ca2+-mediated signaling in time keeping the mitochondrial metabolism and in its feed-back modulation of the circadian clockwork.
中文翻译:

线粒体钙驱动丙酮酸脱氢酶和氧化磷酸化的时钟基因依赖性激活。
代谢的调节正在作为哺乳动物昼夜节律电路的主要输出而出现。因此,线粒体的氧化代谢在体内和体外每天都经历振荡活动。在先前的研究中,我们显示,糖酵解和线粒体耗氧量在同步的HepG2细胞培养物中均显示出类似的时间分辨的节律活动,转化为总体生物能变化,如此处通过测量ATP水平所证明的。用特定的代谢抑制剂处理同步细胞后,丙酮酸成为减少呼吸链等价物的主要来源,其氧化作用是由丙酮酸脱氢酶的有节奏的(去)磷酸化驱动的。2+。线粒体Ca 2+单向转运蛋白抑制剂以及雷诺丹碱受体Ca 2+通道或ADPR环化酶的抑制剂抑制了线粒体呼吸的节律性变化,表明线粒体Ca 2+内流源于ER储存,可能在接触线粒体区室。值得注意的是,线粒体Ca 2+内流的阻塞导致规范时钟基因(如BMALl1,CLOCK和NR1D1)的表达失调。总之,我们的发现揭示了Ca 2+迄今尚未探索的功能介导的信号传递,可以保持线粒体的新陈代谢及其对生物钟发条的反馈调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号