当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-04 , DOI: 10.1039/d0qo00624f Jakob Gaar 1, 2, 3, 4, 5 , Rafea Naffa 4, 6, 7 , Margaret Brimble 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-04 , DOI: 10.1039/d0qo00624f Jakob Gaar 1, 2, 3, 4, 5 , Rafea Naffa 4, 6, 7 , Margaret Brimble 1, 2, 3, 4, 5
Affiliation
|
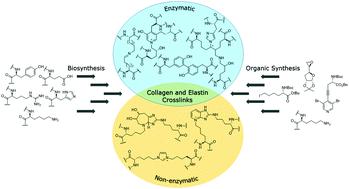
Collagen and elastin are the most abundant structural proteins in animals and play an integral biological and structural role in the extracellular matrix. The biosynthesis and maturation of collagen and elastin occurs via multi-step intracellular and extracellular processes including the formation of several covalent crosslinks to stabilise their structure, confer thermal stability and provide biochemical properties to tissues. There are two major groups of crosslinks based on their formation pathways, enzymatic and non-enzymatic. The biosynthesis of enzymatic crosslinks starts with the enzymatic oxidation of lysine or hydroxylysine residues into aldehydes. These aldehdyes undergo a series of spontaneous condensation reactions with lysine, hydroxylysine or other aldehdye residues to form immature covalent crosslinks which are further matured via poorly understood mechanisms into multivalent crosslinks. While enzymatic crosslinks make up the majority of protein–protein crosslinks, the non-enzymatic unselective glycation of lysine residues via the Maillard reaction results in the formation of Advanced Glycation Endproducts (AGEs). These latter biosynthesis pathways are not fully understood as they are produced by a series of oxidative reactions between carbohydrates and collagen via Amadori rearrangements. Both covalent crosslinks and AGEs appear to correlate with several diseases such as skin and bone disorders, cancer metastasis, diabetes, Alzheimer's and cardiovascular diseases. Although several crosslinks are isolated, purified and described in collagen and elastin, only a few of them are chemically synthesized. Chemical synthesis plays an essential and important role in research providing pure crosslinks as reference materials and enabling the discovery of compounds to understand the biosynthesis of crosslinks and their properties. Synthetic crosslinks are crucial to verify the structures of collagen and elastin crosslinks where only a handful of structures have been determined by NMR spectroscopy and many other structures have only been predicted using mass spectrometry. This makes crosslinks and AGEs an interesting target for organic synthesis to produce sufficient quantities of material to enable studies on their biological significance and determine their absolute stereochemistry. The biological and chemical synthesis of both enzymatic and non-enzymatic crosslinks are extensively described in this review.
中文翻译:

胶原蛋白和弹性蛋白中的酶促和非酶促交联及其化学合成
胶原蛋白和弹性蛋白是动物中最丰富的结构蛋白,在细胞外基质中起着不可或缺的生物学和结构作用。胶原蛋白和弹性蛋白的生物合成和成熟通过多步细胞内和细胞外过程,包括几个共价交联的形成,以稳定其结构,赋予热稳定性并为组织提供生化特性。基于交联的形成途径,主要有两类交联:酶促和非酶促。酶促交联的生物合成始于赖氨酸或羟赖氨酸残基的酶促氧化为醛。这些aldehdyes经历一系列与赖氨酸,羟赖氨酸或其它残基aldehdye自发缩合反应以形成不成熟的共价交联被进一步熟化经由对多价交联的机制了解甚少。尽管酶促交联构成了大部分蛋白质-蛋白交联,但是通过美拉德反应对赖氨酸残基进行非酶促非选择性糖基化会导致高级糖基化终产物(AGEs)的形成。这些后期的生物合成途径尚不完全清楚,因为它们是由碳水化合物和胶原蛋白之间的一系列氧化反应通过Amadori重排。共价交联和AGEs似乎与几种疾病有关,例如皮肤和骨骼疾病,癌症转移,糖尿病,阿尔茨海默氏病和心血管疾病。尽管在胶原蛋白和弹性蛋白中分离,纯化和描述了几种交联键,但只有少数是化学合成的。化学合成在提供纯交联作为参考材料的研究中起着至关重要的作用,并使化合物的发现能够理解交联的生物合成及其性质。合成交联对于验证胶原蛋白和弹性蛋白交联的结构至关重要,胶原蛋白和弹性蛋白交联的结构只有少数结构已通过NMR光谱确定,许多其他结构仅通过质谱法预测。这使得交联和AGEs成为有机合成的有趣目标,以产生足够数量的材料,从而能够对其生物学意义进行研究并确定其绝对立体化学。在这篇综述中广泛地描述了酶和非酶交联的生物和化学合成。
更新日期:2020-09-16
中文翻译:

胶原蛋白和弹性蛋白中的酶促和非酶促交联及其化学合成
胶原蛋白和弹性蛋白是动物中最丰富的结构蛋白,在细胞外基质中起着不可或缺的生物学和结构作用。胶原蛋白和弹性蛋白的生物合成和成熟通过多步细胞内和细胞外过程,包括几个共价交联的形成,以稳定其结构,赋予热稳定性并为组织提供生化特性。基于交联的形成途径,主要有两类交联:酶促和非酶促。酶促交联的生物合成始于赖氨酸或羟赖氨酸残基的酶促氧化为醛。这些aldehdyes经历一系列与赖氨酸,羟赖氨酸或其它残基aldehdye自发缩合反应以形成不成熟的共价交联被进一步熟化经由对多价交联的机制了解甚少。尽管酶促交联构成了大部分蛋白质-蛋白交联,但是通过美拉德反应对赖氨酸残基进行非酶促非选择性糖基化会导致高级糖基化终产物(AGEs)的形成。这些后期的生物合成途径尚不完全清楚,因为它们是由碳水化合物和胶原蛋白之间的一系列氧化反应通过Amadori重排。共价交联和AGEs似乎与几种疾病有关,例如皮肤和骨骼疾病,癌症转移,糖尿病,阿尔茨海默氏病和心血管疾病。尽管在胶原蛋白和弹性蛋白中分离,纯化和描述了几种交联键,但只有少数是化学合成的。化学合成在提供纯交联作为参考材料的研究中起着至关重要的作用,并使化合物的发现能够理解交联的生物合成及其性质。合成交联对于验证胶原蛋白和弹性蛋白交联的结构至关重要,胶原蛋白和弹性蛋白交联的结构只有少数结构已通过NMR光谱确定,许多其他结构仅通过质谱法预测。这使得交联和AGEs成为有机合成的有趣目标,以产生足够数量的材料,从而能够对其生物学意义进行研究并确定其绝对立体化学。在这篇综述中广泛地描述了酶和非酶交联的生物和化学合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号