当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc induced structural changes in the intrinsically disordered BDNF Met prodomain confer synaptic elimination.
Metallomics ( IF 2.9 ) Pub Date : 2020-08-03 , DOI: 10.1039/d0mt00108b Jing Wang 1 , Agustin Anastasia , Henrietta Bains , Joanna I Giza , David G Clossey , Jingjing Deng , Thomas A Neubert , William J Rice , Francis S Lee , Barbara L Hempstead , Clay Bracken
Metallomics ( IF 2.9 ) Pub Date : 2020-08-03 , DOI: 10.1039/d0mt00108b Jing Wang 1 , Agustin Anastasia , Henrietta Bains , Joanna I Giza , David G Clossey , Jingjing Deng , Thomas A Neubert , William J Rice , Francis S Lee , Barbara L Hempstead , Clay Bracken
Affiliation
|
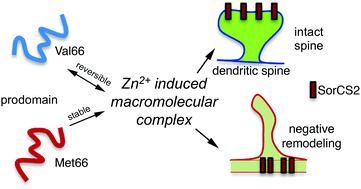
Human brain derived neurotrophic factor (BDNF) encodes a protein product consisting of a C-terminal mature domain (mature BDNF) and an N-terminal prodomain, which is an intrinsically disordered protein. A common single nucleotide polymorphism in humans results in a methionine substitution for valine at position 66 of the prodomain, and is associated with memory deficits, depression and anxiety disorders. The BDNF Met66 prodomain, but not the Val66 prodomain, promotes rapid structural remodeling of hippocampal neurons’ growth cones and dendritic spines by interacting directly with the SorCS2 receptor. While it has been reported that the Met66 and Val66 prodomains exhibit only modest differences in structural propensities in the apo state, here we show that Val66 and Met66 prodomains differentially bind zinc (Zn). Zn2+ binds with higher affinity and more broadly impacts residues on the Met66 prodomain compared to the Val66 prodomain as shown by NMR and ITC. Zn2+ binding to the Met66 and Val66 prodomains results in distinct conformational and macroscopic differences observed by NMR, light scattering and cryoEM. To determine if Zn2+ mediated conformational change in the Met66 prodomain is required for biological effect, we mutated His40, a Zn2+ binding site, and observed a loss of Met66 prodomain bioactivity. As the His40 site is distant from the known region of the prodomain involved in receptor binding, we suggest that Met66 prodomain bioactivity involves His40 mediated stabilization of the multimeric structure. Our results point to the necessity of a Zn2+-mediated higher order molecular assembly of the Met66 prodomain to mediate neuronal remodeling.
中文翻译:

锌在本质上无序的 BDNF Met 前结构域中诱导结构变化,赋予突触消除。
人脑衍生的神经营养因子 (BDNF) 编码的蛋白质产物由 C 端成熟结构域 (成熟 BDNF) 和 N 端前结构域组成,这是一种本质上无序的蛋白质。人类常见的单核苷酸多态性导致前结构域 66 位的缬氨酸被蛋氨酸取代,并与记忆缺陷、抑郁症和焦虑症有关。BDNF Met66 前结构域,但不是 Val66 前结构域,通过直接与 SorCS2 受体相互作用,促进海马神经元生长锥和树突棘的快速结构重塑。虽然据报道 Met66 和 Val66 前结构域在 apo 状态下的结构倾向仅表现出适度的差异,但在这里我们显示 Val66 和 Met66 前结构域差异结合锌 (Zn)。锌2+如 NMR 和 ITC 所示,与 Val66 前结构域相比,Met66 前结构域以更高的亲和力结合并更广泛地影响残基。Zn 2+与 Met66 和 Val66 前结构域的结合导致通过 NMR、光散射和冷冻电镜观察到明显的构象和宏观差异。为了确定 Zn 2+介导的 Met66 前域构象变化是否是生物学效应所必需的,我们突变了 Zn 2+结合位点 His40,并观察到 Met66 前域生物活性的丧失。由于 His40 位点远离参与受体结合的前结构域的已知区域,我们建议 Met66 前结构域生物活性涉及 His40 介导的多聚体结构的稳定化。我们的结果表明 Zn 2+的必要性-介导 Met66 前结构域的高阶分子组装以介导神经元重塑。
更新日期:2020-08-19
中文翻译:

锌在本质上无序的 BDNF Met 前结构域中诱导结构变化,赋予突触消除。
人脑衍生的神经营养因子 (BDNF) 编码的蛋白质产物由 C 端成熟结构域 (成熟 BDNF) 和 N 端前结构域组成,这是一种本质上无序的蛋白质。人类常见的单核苷酸多态性导致前结构域 66 位的缬氨酸被蛋氨酸取代,并与记忆缺陷、抑郁症和焦虑症有关。BDNF Met66 前结构域,但不是 Val66 前结构域,通过直接与 SorCS2 受体相互作用,促进海马神经元生长锥和树突棘的快速结构重塑。虽然据报道 Met66 和 Val66 前结构域在 apo 状态下的结构倾向仅表现出适度的差异,但在这里我们显示 Val66 和 Met66 前结构域差异结合锌 (Zn)。锌2+如 NMR 和 ITC 所示,与 Val66 前结构域相比,Met66 前结构域以更高的亲和力结合并更广泛地影响残基。Zn 2+与 Met66 和 Val66 前结构域的结合导致通过 NMR、光散射和冷冻电镜观察到明显的构象和宏观差异。为了确定 Zn 2+介导的 Met66 前域构象变化是否是生物学效应所必需的,我们突变了 Zn 2+结合位点 His40,并观察到 Met66 前域生物活性的丧失。由于 His40 位点远离参与受体结合的前结构域的已知区域,我们建议 Met66 前结构域生物活性涉及 His40 介导的多聚体结构的稳定化。我们的结果表明 Zn 2+的必要性-介导 Met66 前结构域的高阶分子组装以介导神经元重塑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号