当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hierarchical assembly of dual-responsive biomineralized polydopamine-calcium phosphate nanocomposites for enhancing chemo-photothermal therapy by autophagy inhibition.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-08-03 , DOI: 10.1039/d0bm00142b Xuan Wang 1 , Yunhao Li , Yankun Cui , Xiongwei Deng , Jianqing Lu , Fan Jia , Zian Pan , Xinyue Cui , Fanqi Hu , Wenhao Hu , Xuesong Zhang , Yan Wu
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-08-03 , DOI: 10.1039/d0bm00142b Xuan Wang 1 , Yunhao Li , Yankun Cui , Xiongwei Deng , Jianqing Lu , Fan Jia , Zian Pan , Xinyue Cui , Fanqi Hu , Wenhao Hu , Xuesong Zhang , Yan Wu
Affiliation
|
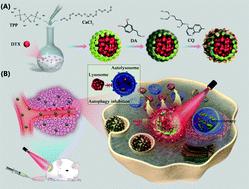
The induction of autophagy in cancer cells would occur in response to several therapy strategies, including chemotherapy and photothermal therapy (PTT). Hence, combined autophagy inhibition has been regarded as a prevailing strategy to enhance treatment sensitivity in cancers. Herein, dual pH/thermal responsive biomineralized nanocomposites (PCNPs) were rationally designed and prepared based on the hierarchical assembly of calcium phosphate (CaP) and polydopamine (PDA). The first step in the self-assembly process involves the incorporation of hydrophobic chemotherapeutic docetaxel (DTX) into the CaP nanoparticles. Next, PDA was utilized as the coating to hierarchically self-assemble onto the surface of CaP through a simple self-polymerization of dopamine. Third, the autophagy inhibitor chloroquine (CQ) was absorbed onto the surface of PDA via non-covalent interactions, forming PCNPs/DC. CQ was the only FDA approved autophagy inhibitor in clinical trials that could inhibit autophagosome fusion and degradation. The resulting PCNPs/DC could exhibit dual pH/thermal responsive properties due to the acid-sensitive CaP core and the photothermal effect of the PDA coating. Effective inhibition of autophagy in cancer cells could be realized by blocking the lysosome and weakening the degradation of autolysosomes by PCNPs/DC. Interestingly, complementary autophagy inhibition could therefore sensitize the effects of chemo-photothermal therapy both in vitro and in vivo with negligible toxicity. Therefore, these hierarchically assembled biomineralized nanocomposites would be used as a prevailing strategy to sensitize chemo-photothermal therapy by complementary autophagy inhibition.
中文翻译:

双重响应的生物矿化的聚多巴胺-磷酸钙纳米复合材料的层次组装,通过自噬抑制作用增强化学光热疗法。
响应包括化学疗法和光热疗法(PTT)在内的几种治疗策略,会发生癌细胞中自噬的诱导。因此,联合自噬抑制被认为是增强癌症治疗敏感性的主要策略。在此,基于磷酸钙(CaP)和聚多巴胺(PDA)的分层组装,合理设计和制备了双重pH /热响应性生物矿化纳米复合材料(PCNP)。自组装过程的第一步涉及将疏水性化疗多西紫杉醇(DTX)掺入CaP纳米颗粒中。接下来,通过多巴胺的简单自聚合,将PDA用作涂层,使其在CaP的表面上分层自组装。第三,自噬抑制剂氯喹(CQ)被吸收到PDA表面通过非共价相互作用形成PCNP / DC。在临床试验中,CQ是唯一获得FDA批准的可抑制自噬体融合和降解的自噬抑制剂。由于酸敏感的CaP核和PDA涂层的光热效应,所得的PCNP / DC可能具有双重pH /热响应特性。通过阻断溶酶体并减弱PCNPs / DC对自溶酶体的降解,可以实现对癌细胞自噬的有效抑制。有趣的是,互补的自噬抑制作用因此可以在体外和体内敏化化学光热疗法的作用。毒性可忽略不计。因此,这些分层组装的生物矿化纳米复合材料将被用作通过互补自噬抑制来敏化化学光热疗法的主流策略。
更新日期:2020-09-15
中文翻译:

双重响应的生物矿化的聚多巴胺-磷酸钙纳米复合材料的层次组装,通过自噬抑制作用增强化学光热疗法。
响应包括化学疗法和光热疗法(PTT)在内的几种治疗策略,会发生癌细胞中自噬的诱导。因此,联合自噬抑制被认为是增强癌症治疗敏感性的主要策略。在此,基于磷酸钙(CaP)和聚多巴胺(PDA)的分层组装,合理设计和制备了双重pH /热响应性生物矿化纳米复合材料(PCNP)。自组装过程的第一步涉及将疏水性化疗多西紫杉醇(DTX)掺入CaP纳米颗粒中。接下来,通过多巴胺的简单自聚合,将PDA用作涂层,使其在CaP的表面上分层自组装。第三,自噬抑制剂氯喹(CQ)被吸收到PDA表面通过非共价相互作用形成PCNP / DC。在临床试验中,CQ是唯一获得FDA批准的可抑制自噬体融合和降解的自噬抑制剂。由于酸敏感的CaP核和PDA涂层的光热效应,所得的PCNP / DC可能具有双重pH /热响应特性。通过阻断溶酶体并减弱PCNPs / DC对自溶酶体的降解,可以实现对癌细胞自噬的有效抑制。有趣的是,互补的自噬抑制作用因此可以在体外和体内敏化化学光热疗法的作用。毒性可忽略不计。因此,这些分层组装的生物矿化纳米复合材料将被用作通过互补自噬抑制来敏化化学光热疗法的主流策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号