当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cleavage of OmpX by protealysin can regulate Serratia proteamaculans invasion
FEBS Letters ( IF 3.0 ) Pub Date : 2020-08-14 , DOI: 10.1002/1873-3468.13897 Olga Tsaplina 1 , Ilya Demidyuk 2 , Tatiana Artamonova 3 , Mikhail Khodorkovsky 3 , Sofia Khaitlina 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-08-14 , DOI: 10.1002/1873-3468.13897 Olga Tsaplina 1 , Ilya Demidyuk 2 , Tatiana Artamonova 3 , Mikhail Khodorkovsky 3 , Sofia Khaitlina 1
Affiliation
|
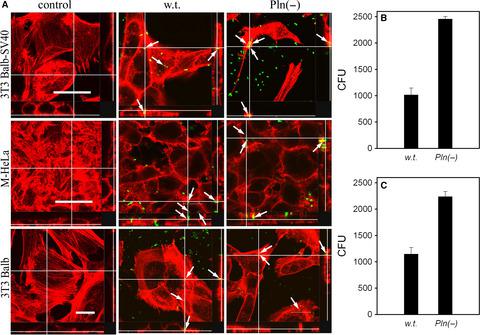
Protealysin is a thermolysin‐like protease of Serratia proteamaculans capable of specifically cleaving actin, which correlates with the invasive activity of these bacteria. Here, we show that inactivation of the protealysin gene does not inhibit invasion but, in contrast, leads to a twofold increase in the S. proteamaculans invasive activity. By mass spectrometry, we identified the outer membrane protein OmpX as a substrate of protealysin. Recombinant E. coli carrying the OmpX gene truncated by 40 N‐terminal residues or both the OmpX and protealysin genes, in contrast to the full‐length OmpX, do not increase adhesion of these bacteria, indicating that the 40 N‐terminal residues of OmpX are indispensable for S. proteamaculans invasion. Our results show that both protealysin and its substrates can stimulate Serratia invasion.
中文翻译:

蛋白溶解素对 OmpX 的裂解可以调节原形沙雷氏菌的入侵
Protealysin 是 Serratia proteamaculans 的嗜热菌蛋白酶样蛋白酶,能够特异性裂解肌动蛋白,这与这些细菌的侵袭活性有关。在这里,我们表明蛋白酶基因的失活不会抑制入侵,但相反,会导致 S. proteamaculans 入侵活动增加两倍。通过质谱,我们确定了外膜蛋白 OmpX 作为蛋白酶的底物。与全长 OmpX 相比,携带被 40 个 N 端残基截断的 OmpX 基因或 OmpX 和溶蛋白基因两者截断的重组大肠杆菌不会增加这些细菌的粘附,表明 OmpX 的 40 个 N 端残基是必不可少的 S. proteamaculans 入侵。我们的结果表明,蛋白酶及其底物都可以刺激沙雷氏菌入侵。
更新日期:2020-08-14
中文翻译:

蛋白溶解素对 OmpX 的裂解可以调节原形沙雷氏菌的入侵
Protealysin 是 Serratia proteamaculans 的嗜热菌蛋白酶样蛋白酶,能够特异性裂解肌动蛋白,这与这些细菌的侵袭活性有关。在这里,我们表明蛋白酶基因的失活不会抑制入侵,但相反,会导致 S. proteamaculans 入侵活动增加两倍。通过质谱,我们确定了外膜蛋白 OmpX 作为蛋白酶的底物。与全长 OmpX 相比,携带被 40 个 N 端残基截断的 OmpX 基因或 OmpX 和溶蛋白基因两者截断的重组大肠杆菌不会增加这些细菌的粘附,表明 OmpX 的 40 个 N 端残基是必不可少的 S. proteamaculans 入侵。我们的结果表明,蛋白酶及其底物都可以刺激沙雷氏菌入侵。











































 京公网安备 11010802027423号
京公网安备 11010802027423号