当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Strategy for Accessing Aldehydes via Palladium‐Catalyzed C−O/C−N Bond Cleavage in the Presence of Hydrosilanes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-03 , DOI: 10.1002/adsc.202000794 Zhanyu He 1 , Zijia Wang 2 , Junxiang Ru 1 , Yulin Wang 1 , Tingting Liu 2 , Zhuo Zeng 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-03 , DOI: 10.1002/adsc.202000794 Zhanyu He 1 , Zijia Wang 2 , Junxiang Ru 1 , Yulin Wang 1 , Tingting Liu 2 , Zhuo Zeng 3
Affiliation
|
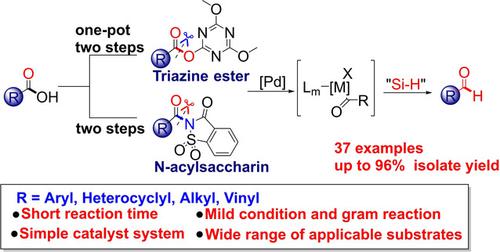
We report the catalytic reduction of both active esters and amides by selective C(acyl)−X (X=O, N) cleavage to access aldehyde functionality via a palladium‐catalyzed strategy. Reactions are promoted by hydrosilanes as reducing reagents with good to excellent yields and with excellent chemoselectivity for C(acyl)−N and C(acyl)−O bond cleavage. Carboxylic acid C(acyl)−O bonds are activated by 2‐chloro‐4,6‐dimethoxy‐1,3,5‐triazine (CDMT) to form triazine ester intermediates, which further react with hydrosilanes to yield aldehydes in one‐pot two‐step procedures. We demonstrate that C(acyl)−O cleavage/formylation offers higher yields and broader substrate scopes compared with C(acyl)−N cleavage under the same reaction conditions.
中文翻译:

在氢硅烷存在下通过钯催化的C-O / C-N键裂解获得醛的策略
我们报告了选择性的C(酰基)-X(X = O,N)裂解,通过钯催化的方法获得醛官能团,从而催化活性酯和酰胺的催化还原。氢化硅烷作为还原剂可以促进反应,其收率好至极好,并且对C(酰基)-N和C(酰基)-O键的裂解具有出色的化学选择性。羧酸的C(酰基)-O键被2-氯-4,6-二甲氧基-1,3,5-三嗪(CDMT)活化形成三嗪酯中间体,该中间体进一步与氢硅烷反应在一个锅中生成醛两步过程。我们证明,与在相同反应条件下进行C(酰基)-N裂解相比,C(酰基)-O裂解/甲酰化可提供更高的收率和更宽的底物范围。
更新日期:2020-08-03
中文翻译:

在氢硅烷存在下通过钯催化的C-O / C-N键裂解获得醛的策略
我们报告了选择性的C(酰基)-X(X = O,N)裂解,通过钯催化的方法获得醛官能团,从而催化活性酯和酰胺的催化还原。氢化硅烷作为还原剂可以促进反应,其收率好至极好,并且对C(酰基)-N和C(酰基)-O键的裂解具有出色的化学选择性。羧酸的C(酰基)-O键被2-氯-4,6-二甲氧基-1,3,5-三嗪(CDMT)活化形成三嗪酯中间体,该中间体进一步与氢硅烷反应在一个锅中生成醛两步过程。我们证明,与在相同反应条件下进行C(酰基)-N裂解相比,C(酰基)-O裂解/甲酰化可提供更高的收率和更宽的底物范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号