当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Approach to α‐ and β‐Amino Peroxides via Lewis Acid Catalyzed Ring Opening‐Peroxidation of Donor‐Acceptor Aziridines and N‐Activated Aziridines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-03 , DOI: 10.1002/adsc.202000815 Kuldeep Singh 1 , Pramod Kumar 1 , Chenna Jagadeesh 1 , Manveer Patel 1 , Dinabandhu Das 2 , Jaideep Saha 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-03 , DOI: 10.1002/adsc.202000815 Kuldeep Singh 1 , Pramod Kumar 1 , Chenna Jagadeesh 1 , Manveer Patel 1 , Dinabandhu Das 2 , Jaideep Saha 1
Affiliation
|
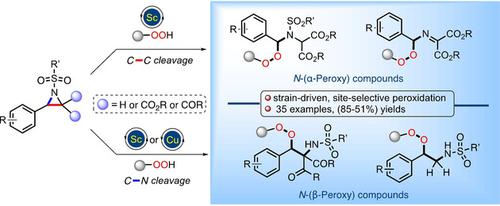
Site‐selective ring‐opening process of two different aziridine classes with hydroperoxide is described herein that provides access to various α‐ and β‐amino and α‐(imino)‐peroxy compounds. This strain‐release‐driven peroxide addition to aziridines represents an alternative approach for entries to biologically significant heteroatom substituted organic peroxides and complements existing methods in the field. The peroxide products obtained by this method displayed a different reactivity during peroxide‐specific rearrangement processes promoted by either acid or base. Mechanistic studies and useful synthetic elaboration of the products have also been presented.
中文翻译:

路易斯酸催化供体-受体氮丙啶和N-活化氮丙啶的开环-过氧化反应制备α-和β-氨基过氧化物
本文介绍了两种不同的氮丙啶类化合物与氢过氧化物的定点开环过程,可提供各种α-和β-氨基以及α-(亚氨基)-过氧化合物的进入途径。这种由应变释放驱动的过氧化物加到氮丙啶中代表了进入生物学上重要的杂原子取代的有机过氧化物的替代方法,并补充了该领域中现有的方法。通过这种方法获得的过氧化物产物在由酸或碱促进的过氧化物特异性重排过程中显示出不同的反应性。还介绍了产品的机理研究和有用的合成工艺。
更新日期:2020-10-06
中文翻译:

路易斯酸催化供体-受体氮丙啶和N-活化氮丙啶的开环-过氧化反应制备α-和β-氨基过氧化物
本文介绍了两种不同的氮丙啶类化合物与氢过氧化物的定点开环过程,可提供各种α-和β-氨基以及α-(亚氨基)-过氧化合物的进入途径。这种由应变释放驱动的过氧化物加到氮丙啶中代表了进入生物学上重要的杂原子取代的有机过氧化物的替代方法,并补充了该领域中现有的方法。通过这种方法获得的过氧化物产物在由酸或碱促进的过氧化物特异性重排过程中显示出不同的反应性。还介绍了产品的机理研究和有用的合成工艺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号