当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Attenuation of epigenetic regulator SMARCA4 and ERK-ETS signaling suppresses aging-related dopaminergic degeneration.
Aging Cell ( IF 8.0 ) Pub Date : 2020-08-04 , DOI: 10.1111/acel.13210 Ling Sun 1 , Jie Zhang 2 , Wenfeng Chen 1 , Yun Chen 1 , Xiaohui Zhang 1 , Mingjuan Yang 1 , Min Xiao 1 , Fujun Ma 1 , Yizhou Yao 1 , Meina Ye 1 , Zhenkun Zhang 1 , Kai Chen 1 , Fei Chen 1 , Yujun Ren 1 , Shiwei Ni 1 , Xi Zhang 1 , Zhangming Yan 3 , Zhi-Rong Sun 3 , Hai-Meng Zhou 4 , Hongqin Yang 5 , Shusen Xie 5 , M Emdadul Haque 6 , Kun Huang 1, 7 , Yufeng Yang 1, 5
Aging Cell ( IF 8.0 ) Pub Date : 2020-08-04 , DOI: 10.1111/acel.13210 Ling Sun 1 , Jie Zhang 2 , Wenfeng Chen 1 , Yun Chen 1 , Xiaohui Zhang 1 , Mingjuan Yang 1 , Min Xiao 1 , Fujun Ma 1 , Yizhou Yao 1 , Meina Ye 1 , Zhenkun Zhang 1 , Kai Chen 1 , Fei Chen 1 , Yujun Ren 1 , Shiwei Ni 1 , Xi Zhang 1 , Zhangming Yan 3 , Zhi-Rong Sun 3 , Hai-Meng Zhou 4 , Hongqin Yang 5 , Shusen Xie 5 , M Emdadul Haque 6 , Kun Huang 1, 7 , Yufeng Yang 1, 5
Affiliation
|
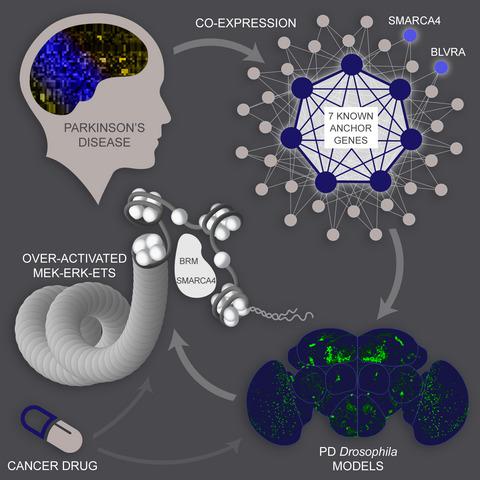
How complex interactions of genetic, environmental factors and aging jointly contribute to dopaminergic degeneration in Parkinson's disease (PD) is largely unclear. Here, we applied frequent gene co‐expression analysis on human patient substantia nigra‐specific microarray datasets to identify potential novel disease‐related genes. In vivo Drosophila studies validated two of 32 candidate genes, a chromatin‐remodeling factor SMARCA4 and a biliverdin reductase BLVRA. Inhibition of SMARCA4 was able to prevent aging‐dependent dopaminergic degeneration not only caused by overexpression of BLVRA but also in four most common Drosophila PD models. Furthermore, down‐regulation of SMARCA4 specifically in the dopaminergic neurons prevented shortening of life span caused by α‐synuclein and LRRK2. Mechanistically, aberrant SMARCA4 and BLVRA converged on elevated ERK‐ETS activity, attenuation of which by either genetic or pharmacological manipulation effectively suppressed dopaminergic degeneration in Drosophila in vivo. Down‐regulation of SMARCA4 or drug inhibition of MEK/ERK also mitigated mitochondrial defects in PINK1 (a PD‐associated gene)‐deficient human cells. Our findings underscore the important role of epigenetic regulators and implicate a common signaling axis for therapeutic intervention in normal aging and a broad range of age‐related disorders including PD.
中文翻译:

表观遗传调节因子 SMARCA4 和 ERK-ETS 信号的减弱抑制了与衰老相关的多巴胺能变性。
遗传、环境因素和衰老的复杂相互作用如何共同导致帕金森病 (PD) 的多巴胺能变性尚不清楚。在这里,我们对人类患者黑质特异性微阵列数据集应用频繁的基因共表达分析,以识别潜在的新型疾病相关基因。体内果蝇研究验证了 32 个候选基因中的两个,染色质重塑因子 SMARCA4 和胆绿素还原酶 BLVRA。抑制 SMARCA4 能够防止衰老依赖性多巴胺能变性不仅由 BLVRA 过度表达引起,而且在四种最常见的果蝇中也能预防PD 模型。此外,多巴胺能神经元中 SMARCA4 的下调可防止由 α-突触核蛋白和 LRRK2 引起的寿命缩短。从机制上讲,异常的 SMARCA4 和 BLVRA 集中在升高的 ERK-ETS 活性上,通过遗传或药理学操作的减弱有效抑制了体内果蝇的多巴胺能变性。SMARCA4 的下调或 MEK/ERK 的药物抑制也减轻了PINK1(一种 PD 相关基因)缺陷的人类细胞中的线粒体缺陷。我们的研究结果强调了表观遗传调节因子的重要作用,并暗示了对正常衰老和包括 PD 在内的广泛年龄相关疾病进行治疗干预的共同信号轴。
更新日期:2020-09-24
中文翻译:

表观遗传调节因子 SMARCA4 和 ERK-ETS 信号的减弱抑制了与衰老相关的多巴胺能变性。
遗传、环境因素和衰老的复杂相互作用如何共同导致帕金森病 (PD) 的多巴胺能变性尚不清楚。在这里,我们对人类患者黑质特异性微阵列数据集应用频繁的基因共表达分析,以识别潜在的新型疾病相关基因。体内果蝇研究验证了 32 个候选基因中的两个,染色质重塑因子 SMARCA4 和胆绿素还原酶 BLVRA。抑制 SMARCA4 能够防止衰老依赖性多巴胺能变性不仅由 BLVRA 过度表达引起,而且在四种最常见的果蝇中也能预防PD 模型。此外,多巴胺能神经元中 SMARCA4 的下调可防止由 α-突触核蛋白和 LRRK2 引起的寿命缩短。从机制上讲,异常的 SMARCA4 和 BLVRA 集中在升高的 ERK-ETS 活性上,通过遗传或药理学操作的减弱有效抑制了体内果蝇的多巴胺能变性。SMARCA4 的下调或 MEK/ERK 的药物抑制也减轻了PINK1(一种 PD 相关基因)缺陷的人类细胞中的线粒体缺陷。我们的研究结果强调了表观遗传调节因子的重要作用,并暗示了对正常衰老和包括 PD 在内的广泛年龄相关疾病进行治疗干预的共同信号轴。











































 京公网安备 11010802027423号
京公网安备 11010802027423号