当前位置:
X-MOL 学术
›
J. Food Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel peroxidase from runner bean (Phaseolus coccineus L.): Enhanced affinity purification, characterization, and dye decolorization activity.
Journal of Food Biochemistry ( IF 3.5 ) Pub Date : 2020-08-03 , DOI: 10.1111/jfbc.13411 Aykut Oztekin 1 , Seyma Tasbasi 2
Journal of Food Biochemistry ( IF 3.5 ) Pub Date : 2020-08-03 , DOI: 10.1111/jfbc.13411 Aykut Oztekin 1 , Seyma Tasbasi 2
Affiliation
|
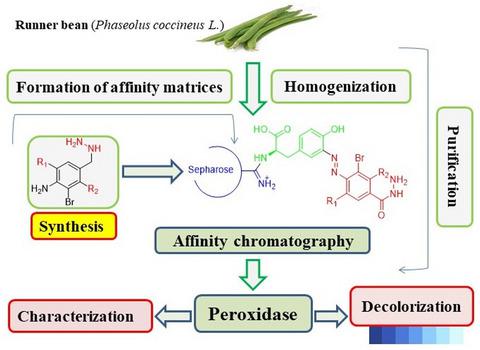
In this study, a novel runner bean peroxidase (RBP) was purified and characterized. Affinity‐based purification was performed with newly synthesized disubstituted 4‐aminobenzohydrazides. In the purification results, 253‐fold was achieved with a yield of 56.2%. Furthermore, molecular weight and enzyme purity were checked with the SDS‐PAGE and observed a single band at 31.2 kDa. Optimum conditions were determined as temperature = 50°C, ionic strength = 0.2 M, and pH 7.0. Enzyme exhibited 31.2% of residual activity in the presence of 20% DMSO. Additionally, the redox‐mediated decolorization effect of the enzyme was examined for Reactive Blue 19 and Acid Blue 25 dyes. As a result of 1‐hr incubation, the enzyme removal activity of Reactive Blue 19 and Acid Blue 25 dyes was calculated as 47% and 57%, respectively.
中文翻译:

一种来自红花菜豆(Phaseolus coccineus L.)的新型过氧化物酶:增强亲和纯化、表征和染料脱色活性。
在这项研究中,纯化并表征了一种新型花菜豆过氧化物酶(RBP)。使用新合成的二取代 4-氨基苯甲酰肼进行基于亲和力的纯化。纯化结果达到253倍,收率56.2%。此外,使用 SDS-PAGE 检查分子量和酶纯度,并观察到 31.2 kDa 处的单条带。最佳条件确定为温度= 50°C,离子强度= 0.2 M,pH 7.0。在 20% DMSO 存在下,酶表现出 31.2% 的残余活性。此外,还检查了酶对活性蓝 19 和酸性蓝 25 染料的氧化还原介导的脱色效果。孵育 1 小时的结果是,活性蓝 19 和酸性蓝 25 染料的酶去除活性分别为 47% 和 57%。
更新日期:2020-10-11
中文翻译:

一种来自红花菜豆(Phaseolus coccineus L.)的新型过氧化物酶:增强亲和纯化、表征和染料脱色活性。
在这项研究中,纯化并表征了一种新型花菜豆过氧化物酶(RBP)。使用新合成的二取代 4-氨基苯甲酰肼进行基于亲和力的纯化。纯化结果达到253倍,收率56.2%。此外,使用 SDS-PAGE 检查分子量和酶纯度,并观察到 31.2 kDa 处的单条带。最佳条件确定为温度= 50°C,离子强度= 0.2 M,pH 7.0。在 20% DMSO 存在下,酶表现出 31.2% 的残余活性。此外,还检查了酶对活性蓝 19 和酸性蓝 25 染料的氧化还原介导的脱色效果。孵育 1 小时的结果是,活性蓝 19 和酸性蓝 25 染料的酶去除活性分别为 47% 和 57%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号