当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Addition of Water, Alcohols, and Amines in Palladium Catalysis.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-03 , DOI: 10.1002/anie.202008350 Annette Grünwald 1 , Frank W Heinemann 1 , Dominik Munz 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-03 , DOI: 10.1002/anie.202008350 Annette Grünwald 1 , Frank W Heinemann 1 , Dominik Munz 1, 2
Affiliation
|
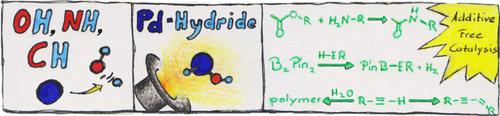
The homolytic cleavage of O−H and N−H or weak C−H bonds is a key elementary step in redox catalysis, but is thought to be unfeasible for palladium. In stark contrast, reported here is the room temperature and reversible oxidative addition of water, isopropanol, hexafluoroisopropanol, phenol, and aniline to a palladium(0) complex with a cyclic (alkyl)(amino)carbene (CAAC) and a labile pyridino ligand, as is also the case in popular N‐heterocyclic carbene (NHC) palladium(II) precatalysts. The oxidative addition of protic solvents or adventitious water switches the chemoselectivity in catalysis with alkynes through activation of the terminal C−H bond. Most salient, the homolytic activation of alcohols and amines allows atom‐efficient, additive‐free cross‐coupling and transfer hydrogenation under mild reaction conditions with usually unreactive, yet desirable reagents, including esters and bis(pinacolato)diboron.
中文翻译:

钯催化中水、醇和胺的氧化加成。
O−H 和 N−H 或弱 C−H 键的均裂是氧化还原催化中的关键基本步骤,但被认为对于钯来说是不可行的。与此形成鲜明对比的是,本文报道了水、异丙醇、六氟异丙醇、苯酚和苯胺在室温下可逆氧化加成到具有环状(烷基)(氨基)卡宾(CAAC)和不稳定吡啶配体的钯(0)络合物,流行的 N-杂环卡宾 (NHC) 钯 (II) 预催化剂也是如此。质子溶剂或外来水的氧化加成通过激活末端 C−H 键来改变炔烃催化的化学选择性。最重要的是,醇和胺的均解活化允许在温和的反应条件下与通常不反应但理想的试剂(包括酯和双(频哪醇)二硼)进行原子效率高、无添加剂的交叉偶联和转移氢化。
更新日期:2020-08-03
中文翻译:

钯催化中水、醇和胺的氧化加成。
O−H 和 N−H 或弱 C−H 键的均裂是氧化还原催化中的关键基本步骤,但被认为对于钯来说是不可行的。与此形成鲜明对比的是,本文报道了水、异丙醇、六氟异丙醇、苯酚和苯胺在室温下可逆氧化加成到具有环状(烷基)(氨基)卡宾(CAAC)和不稳定吡啶配体的钯(0)络合物,流行的 N-杂环卡宾 (NHC) 钯 (II) 预催化剂也是如此。质子溶剂或外来水的氧化加成通过激活末端 C−H 键来改变炔烃催化的化学选择性。最重要的是,醇和胺的均解活化允许在温和的反应条件下与通常不反应但理想的试剂(包括酯和双(频哪醇)二硼)进行原子效率高、无添加剂的交叉偶联和转移氢化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号