Cell Stem Cell ( IF 19.8 ) Pub Date : 2020-08-04 , DOI: 10.1016/j.stem.2020.07.008 Sepideh Abbasi 1 , Sarthak Sinha 1 , Elodie Labit 1 , Nicole L Rosin 1 , Grace Yoon 1 , Waleed Rahmani 1 , Arzina Jaffer 1 , Nilesh Sharma 1 , Andrew Hagner 1 , Prajay Shah 1 , Rohit Arora 1 , Jessica Yoon 1 , Anowara Islam 1 , Aya Uchida 1 , Chih Kai Chang 2 , Jo Anne Stratton 1 , R Wilder Scott 2 , Fabio M V Rossi 2 , T Michael Underhill 2 , Jeff Biernaskie 3
|
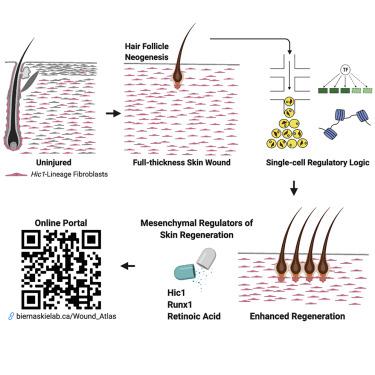
Dermal fibroblasts exhibit considerable heterogeneity during homeostasis and in response to injury. Defining lineage origins of reparative fibroblasts and regulatory programs that drive fibrosis or, conversely, promote regeneration will be essential for improving healing outcomes. Using complementary fate-mapping approaches, we show that hair follicle mesenchymal progenitors make limited contributions to wound repair. In contrast, extrafollicular progenitors marked by the quiescence-associated factor Hic1 generated the bulk of reparative fibroblasts and exhibited functional divergence, mediating regeneration in the center of the wound neodermis and scar formation in the periphery. Single-cell RNA-seq revealed unique transcriptional, regulatory, and epithelial-mesenchymal crosstalk signatures that enabled mesenchymal competence for regeneration. Integration with scATAC-seq highlighted changes in chromatin accessibility within regeneration-associated loci. Finally, pharmacological modulation of RUNX1 and retinoic acid signaling or genetic deletion of Hic1 within wound-activated fibroblasts was sufficient to modulate healing outcomes, suggesting that reparative fibroblasts have latent but modifiable regenerative capacity.
中文翻译:

不同的管理程序控制伤口愈合过程中皮肤成纤维细胞的潜在再生潜能。
真皮成纤维细胞在体内平衡过程中以及对损伤的反应中表现出明显的异质性。定义修复性成纤维细胞的血统起源和驱动纤维化或相反地促进再生的调控程序,对于改善愈合结果至关重要。使用互补的命运映射方法,我们表明毛囊间充质祖细胞对伤口修复的贡献有限。相反,以静止相关因子Hic1为标记的小泡前祖细胞产生了大量的修复性成纤维细胞,并表现出功能性差异,介导伤口新生皮中心的再生和周围瘢痕的形成。单细胞RNA-seq揭示了独特的转录,调节和上皮-间充质串扰信号,使间充质能够再生。与scATAC-seq的整合突出显示了再生相关基因座内染色质可及性的变化。最后,在伤口活化的成纤维细胞内,RUNX1和视黄酸信号的药理学调节或Hic1的基因缺失足以调节愈合结果,表明修复性成纤维细胞具有潜在但可改变的再生能力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号