当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential for the Formation of N-Nitrosamines during the Manufacture of Active Pharmaceutical Ingredients: An Assessment of the Risk Posed by Trace Nitrite in Water
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-03 , DOI: 10.1021/acs.oprd.0c00224 Ian W. Ashworth 1 , Olivier Dirat 2 , Andrew Teasdale 1 , Matthew Whiting 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-03 , DOI: 10.1021/acs.oprd.0c00224 Ian W. Ashworth 1 , Olivier Dirat 2 , Andrew Teasdale 1 , Matthew Whiting 3
Affiliation
|
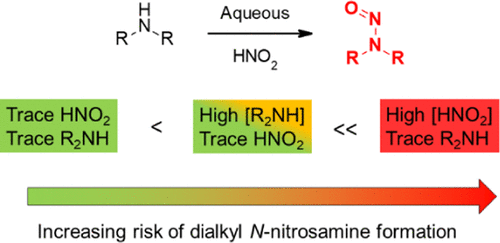
Regulatory requests that marketing authorization holders for chemically synthesized active substances risk assess their medicines for the potential presence of N-nitrosamines have led to a renewed interest in amine nitrosation. We have used published mechanistic and kinetic studies of amine nitrosation to assess the risk that traces of nitrite in the water used during active pharmaceutical ingredient (API) manufacturing could give rise to significant levels of N-nitrosamines. We conclude that the levels of nitrite typically found in water used for API manufacture are very low (<0.01 mg/L) and will not give rise to significant levels of N-nitrosamines through reaction with basic secondary amines (pKa > 9.5) in the majority of cases. The use of less basic amines, elevated processing temperatures, or low pH conditions in combination with elevated levels of nitrite have the potential to generate levels of N-nitrosamines that could lead to significant quantities being present in the isolated API if the downstream processing does not provide an adequate purge. The kinetic models described may be used to risk assess specific situations or processes. For example, the addition of traces of dimethylamine to a nitrosation reaction is predicted to lead to the rapid, quantitative formation of N-nitroso dimethylamine. Simple tertiary alkylamines can nitrosate via a dealkylative process, which is significantly slower than secondary amine nitrosation. Therefore, they do not represent a risk of N-nitrosamine formation under conditions where there is no significant risk of secondary amine nitrosation.
中文翻译:

在制造活性药物成分过程中形成N-亚硝胺的潜力:水中痕量亚硝酸盐构成的风险评估
监管部门要求,化学合成活性物质的销售授权持有人应评估其药物的N-亚硝胺的潜在含量,从而引起人们对胺亚硝化的新兴趣。我们已经使用已发表的有关胺亚硝化的机理和动力学研究,来评估在生产活性药物成分(API)的过程中所使用的水中痕量亚硝酸盐可能导致大量N-亚硝胺的风险。我们得出的结论是,通常用于API生产的水中常见的亚硝酸盐含量非常低(<0.01 mg / L),并且不会通过与碱性仲胺反应而产生显着水平的N-亚硝胺(p K a> 9.5)。如果使用碱性较低的胺,升高的加工温度或较低的pH条件以及较高的亚硝酸盐含量,则有可能生成N-亚硝胺的水平,如果下游加工不进行,则可能导致大量的N-亚硝胺存在于分离的API中。提供足够的净化。所描述的动力学模型可用于风险评估特定情况或过程。例如,预计将痕量的二甲胺添加到亚硝化反应中会导致快速,定量地形成N-亚硝基二甲胺。简单的叔烷基胺可以通过脱烷基过程进行亚硝化,该过程明显比仲胺的亚硝化慢。因此,它们不构成以下风险:在没有明显的仲胺亚硝化风险的条件下形成N-亚硝胺。
更新日期:2020-09-20
中文翻译:

在制造活性药物成分过程中形成N-亚硝胺的潜力:水中痕量亚硝酸盐构成的风险评估
监管部门要求,化学合成活性物质的销售授权持有人应评估其药物的N-亚硝胺的潜在含量,从而引起人们对胺亚硝化的新兴趣。我们已经使用已发表的有关胺亚硝化的机理和动力学研究,来评估在生产活性药物成分(API)的过程中所使用的水中痕量亚硝酸盐可能导致大量N-亚硝胺的风险。我们得出的结论是,通常用于API生产的水中常见的亚硝酸盐含量非常低(<0.01 mg / L),并且不会通过与碱性仲胺反应而产生显着水平的N-亚硝胺(p K a> 9.5)。如果使用碱性较低的胺,升高的加工温度或较低的pH条件以及较高的亚硝酸盐含量,则有可能生成N-亚硝胺的水平,如果下游加工不进行,则可能导致大量的N-亚硝胺存在于分离的API中。提供足够的净化。所描述的动力学模型可用于风险评估特定情况或过程。例如,预计将痕量的二甲胺添加到亚硝化反应中会导致快速,定量地形成N-亚硝基二甲胺。简单的叔烷基胺可以通过脱烷基过程进行亚硝化,该过程明显比仲胺的亚硝化慢。因此,它们不构成以下风险:在没有明显的仲胺亚硝化风险的条件下形成N-亚硝胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号