当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Silicon–Hydrogen Exchange Reaction: A Catalytic σ-Bond Metathesis Approach to the Enantioselective Synthesis of Enol Silanes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-03 , DOI: 10.1021/jacs.0c06677 Hui Zhou 1 , Han Yong Bae 1, 2 , Markus Leutzsch 1 , Jennifer L Kennemur 1 , Diane Bécart 1 , Benjamin List 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-03 , DOI: 10.1021/jacs.0c06677 Hui Zhou 1 , Han Yong Bae 1, 2 , Markus Leutzsch 1 , Jennifer L Kennemur 1 , Diane Bécart 1 , Benjamin List 1
Affiliation
|
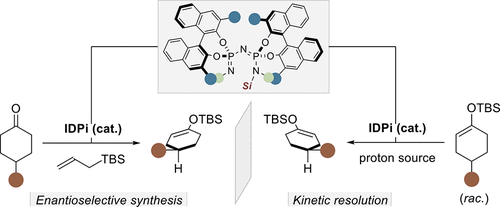
The use of chiral enol silanes in fundamental transformations such as Mukaiyama aldol, Michael, and Mannich reactions as well as Saegusa–Ito dehydrogenations has enabled the chemical synthesis of enantiopure natural products and valuable pharmaceuticals. However, accessing these intermediates in high enantiopurity has generally required the use of either stoichiometric chiral precursors or stoichiometric chiral reagents. We now describe a catalytic approach in which strongly acidic and confined imidodiphosphorimidates (IDPi) catalyze highly enantioselective interconversions of ketones and enol silanes. These “silicon–hydrogen exchange reactions” enable access to enantiopure enol silanes via tautomerizing σ-bond metatheses, either in a deprotosilylative desymmetrization of ketones with allyl silanes as the silicon source or in a protodesilylative kinetic resolution of racemic enol silanes with a carboxylic acid as the silyl acceptor.
中文翻译:

硅氢交换反应:一种对映选择性合成烯醇硅烷的催化 σ-键复分解方法
手性烯醇硅烷在 Mukaiyama aldol、Michael 和 Mannich 反应以及 Saegusa-Ito 脱氢等基本转化中的使用使对映纯天然产物和有价值的药物的化学合成成为可能。然而,以高对映体纯度获取这些中间体通常需要使用化学计量的手性前体或化学计量的手性试剂。我们现在描述一种催化方法,其中强酸性和受限的亚胺二磷酰亚胺 (IDPi) 催化酮和烯醇硅烷的高度对映选择性互变。这些“硅 - 氢交换反应”能够通过互变异构化 σ 键复分解反应获得对映纯烯醇硅烷,
更新日期:2020-08-03
中文翻译:

硅氢交换反应:一种对映选择性合成烯醇硅烷的催化 σ-键复分解方法
手性烯醇硅烷在 Mukaiyama aldol、Michael 和 Mannich 反应以及 Saegusa-Ito 脱氢等基本转化中的使用使对映纯天然产物和有价值的药物的化学合成成为可能。然而,以高对映体纯度获取这些中间体通常需要使用化学计量的手性前体或化学计量的手性试剂。我们现在描述一种催化方法,其中强酸性和受限的亚胺二磷酰亚胺 (IDPi) 催化酮和烯醇硅烷的高度对映选择性互变。这些“硅 - 氢交换反应”能够通过互变异构化 σ 键复分解反应获得对映纯烯醇硅烷,











































 京公网安备 11010802027423号
京公网安备 11010802027423号