当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel structure of the N-terminal helical domain of BibA, a group B streptococcus immunogenic bacterial adhesin.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-08-03 , DOI: 10.1107/s2059798320008116 Kartik Manne 1 , Debasish Chattopadhyay 2 , Vaibhav Agarwal 3 , Anna M Blom 3 , Baldeep Khare 4 , Srinivas Chakravarthy 5 , Chungyu Chang 6 , Hung Ton-That 6 , Sthanam V L Narayana 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-08-03 , DOI: 10.1107/s2059798320008116 Kartik Manne 1 , Debasish Chattopadhyay 2 , Vaibhav Agarwal 3 , Anna M Blom 3 , Baldeep Khare 4 , Srinivas Chakravarthy 5 , Chungyu Chang 6 , Hung Ton-That 6 , Sthanam V L Narayana 1
Affiliation
|
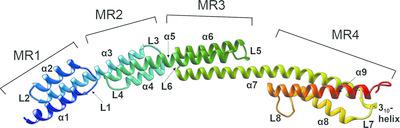
BibA, a group B streptococcus (GBS) surface protein, has been shown to protect the pathogen from phagocytic killing by sequestering a complement inhibitor: C4b‐binding protein (C4BP). Here, the X‐ray crystallographic structure of a GBS BibA fragment (BibA126–398) and a low‐resolution small‐angle X‐ray scattering (SAXS) structure of the full‐length N‐terminal domain (BibA34–400) are described. The BibA126–398 fragment crystal structure displayed a novel and predominantly helical structure. The tertiary arrangement of helices forms four antiparallel three‐helix‐bundle‐motif repeats, with one long helix from a bundle extending into the next. Multiple mutations on recombinant BibA34–400 delayed the degradation of the protein, and circular dichroism spectroscopy of BibA34–400 suggested a similar secondary‐structure composition to that observed in the crystallized BibA126–398 fragment. A model was generated for the 92 N‐terminal residues (BibA34–125) using structural similarity prediction programs, and a BibA34–400 model was generated by combining the coordinates of BibA34–126 and BibA126–398. The X‐ray structure of BibA126–398 and the model of BibA34–400 fitted well into the calculated SAXS envelope. One possible binding site for the BibA N‐terminal domain was localized to the N‐terminal CCP (complement‐control protein) domains of the C4BP α‐chain, as indicated by the decreased binding of BibA to a ΔCCP1 C4BP α‐chain mutant. In summary, it is suggested that the GBS surface protein BibA, which consists of three antiparallel α‐helical‐bundle motifs, is unique and belongs to a new class of Gram‐positive surface adhesins.
中文翻译:

BibA(一种 B 族链球菌免疫原性细菌粘附素)N 端螺旋结构域的新结构。
BibA 是一种 B 族链球菌 (GBS) 表面蛋白,已被证明可以通过隔离补体抑制剂:C4b 结合蛋白 (C4BP) 来保护病原体免遭吞噬杀灭。此处,GBS BibA 片段 (BibA 126–398 ) 的 X 射线晶体结构和全长 N 端结构域 (BibA 34–400 ) 的低分辨率小角度 X 射线散射 (SAXS) 结构进行了描述。 BibA 126-398片段晶体结构显示出一种新颖的、以螺旋为主的结构。螺旋的三级排列形成四个反向平行的三螺旋束基序重复,其中一个长螺旋从一个束延伸到下一个。重组 BibA 34-400上的多个突变延迟了蛋白质的降解,BibA 34-400的圆二色光谱表明其二级结构组成与结晶 BibA 126-398片段中观察到的相似。使用结构相似性预测程序为 92 个 N 末端残基 (BibA 34-125 ) 生成模型,并通过组合 BibA 34-126和 BibA 126-398的坐标生成 BibA 34-400模型。 BibA 126-398的 X 射线结构和 BibA 34-400的模型很好地符合计算的 SAXS 包络线。 BibA N 端结构域的一个可能的结合位点位于 C4BP α 链的 N 端 CCP(补体控制蛋白)结构域,BibA 与 ΔCCP1 C4BP α 链突变体的结合减少表明了这一点。 总之,GBS表面蛋白BibA由三个反向平行的α螺旋束基序组成,是独特的,属于一类新的革兰氏阳性表面粘附素。
更新日期:2020-08-03
中文翻译:

BibA(一种 B 族链球菌免疫原性细菌粘附素)N 端螺旋结构域的新结构。
BibA 是一种 B 族链球菌 (GBS) 表面蛋白,已被证明可以通过隔离补体抑制剂:C4b 结合蛋白 (C4BP) 来保护病原体免遭吞噬杀灭。此处,GBS BibA 片段 (BibA 126–398 ) 的 X 射线晶体结构和全长 N 端结构域 (BibA 34–400 ) 的低分辨率小角度 X 射线散射 (SAXS) 结构进行了描述。 BibA 126-398片段晶体结构显示出一种新颖的、以螺旋为主的结构。螺旋的三级排列形成四个反向平行的三螺旋束基序重复,其中一个长螺旋从一个束延伸到下一个。重组 BibA 34-400上的多个突变延迟了蛋白质的降解,BibA 34-400的圆二色光谱表明其二级结构组成与结晶 BibA 126-398片段中观察到的相似。使用结构相似性预测程序为 92 个 N 末端残基 (BibA 34-125 ) 生成模型,并通过组合 BibA 34-126和 BibA 126-398的坐标生成 BibA 34-400模型。 BibA 126-398的 X 射线结构和 BibA 34-400的模型很好地符合计算的 SAXS 包络线。 BibA N 端结构域的一个可能的结合位点位于 C4BP α 链的 N 端 CCP(补体控制蛋白)结构域,BibA 与 ΔCCP1 C4BP α 链突变体的结合减少表明了这一点。 总之,GBS表面蛋白BibA由三个反向平行的α螺旋束基序组成,是独特的,属于一类新的革兰氏阳性表面粘附素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号