当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aromatic Linkers Unleash the Antiproliferative Potential of 3-Chloropiperidines Against Pancreatic Cancer Cells.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-08-03 , DOI: 10.1002/cmdc.202000457 Tim Helbing 1 , Caterina Carraro 2 , Alexander Francke 1 , Alice Sosic 2 , Michele De Franco 2 , Valentina Gandin 2 , Richard Göttlich 1 , Barbara Gatto 2
ChemMedChem ( IF 3.6 ) Pub Date : 2020-08-03 , DOI: 10.1002/cmdc.202000457 Tim Helbing 1 , Caterina Carraro 2 , Alexander Francke 1 , Alice Sosic 2 , Michele De Franco 2 , Valentina Gandin 2 , Richard Göttlich 1 , Barbara Gatto 2
Affiliation
|
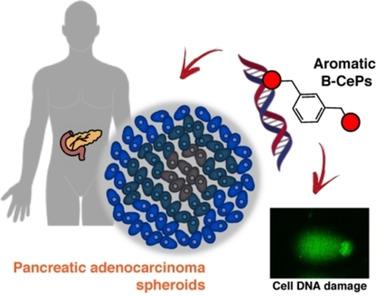
In this study, we describe the synthesis and biological evaluation of a set of bis‐3‐chloropiperidines (B−CePs) containing rigid aromatic linker structures. A modification of the synthetic strategy also enabled the synthesis of a pilot tris‐3‐chloropiperidine (Tri‐CeP) bearing three reactive meta‐chloropiperidine moieties on the aromatic scaffold. A structure–reactivity relationship analysis of B−CePs suggests that the arrangement of the reactive units affects the DNA alkylating activity, while also revealing correlations between the electron density of the aromatic system and the reactivity with biologically relevant nucleophiles, both on isolated DNA and in cancer cells. Interestingly, all aromatic 3‐chloropiperidines exhibited a marked cytotoxicity and tropism for 2D and 3D cultures of pancreatic cancer cells. Therefore, the new aromatic 3‐chloropiperidines appear to be promising contenders for further development of mustard‐based anticancer agents aimed at pancreatic cancers.
中文翻译:

芳香族接头释放 3-氯哌啶抗胰腺癌细胞的抗增殖潜力。
在这项研究中,我们描述了一组含有刚性芳香族连接结构的双-3-氯哌啶(B-CePs)的合成和生物学评价。对合成策略的修改还使得能够合成在芳香族支架上带有三个反应性间氯哌啶部分的试点三-3-氯哌啶(Tri-CeP) 。B−CePs 的结构-反应性关系分析表明,反应单元的排列会影响 DNA 烷基化活性,同时还揭示了芳香系统的电子密度与生物相关亲核试剂的反应性之间的相关性,无论是在分离的 DNA 上还是在癌细胞。有趣的是,所有芳香族 3-氯哌啶对胰腺癌细胞的 2D 和 3D 培养物均表现出显着的细胞毒性和趋向性。因此,新型芳香族3-氯哌啶似乎是进一步开发针对胰腺癌的芥子类抗癌药物的有希望的竞争者。
更新日期:2020-08-03
中文翻译:

芳香族接头释放 3-氯哌啶抗胰腺癌细胞的抗增殖潜力。
在这项研究中,我们描述了一组含有刚性芳香族连接结构的双-3-氯哌啶(B-CePs)的合成和生物学评价。对合成策略的修改还使得能够合成在芳香族支架上带有三个反应性间氯哌啶部分的试点三-3-氯哌啶(Tri-CeP) 。B−CePs 的结构-反应性关系分析表明,反应单元的排列会影响 DNA 烷基化活性,同时还揭示了芳香系统的电子密度与生物相关亲核试剂的反应性之间的相关性,无论是在分离的 DNA 上还是在癌细胞。有趣的是,所有芳香族 3-氯哌啶对胰腺癌细胞的 2D 和 3D 培养物均表现出显着的细胞毒性和趋向性。因此,新型芳香族3-氯哌啶似乎是进一步开发针对胰腺癌的芥子类抗癌药物的有希望的竞争者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号