当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Soybean Oil Hydrolysis on Niobium Catalysts
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-08-02 , DOI: 10.1002/ceat.201900609 Samia Tássia Andrade Maciel 1 , Alexander Andrey Lopes da Silva 2 , Yasmin Guimarães Pedro 1 , Cristiano Nunes da Silva 2 , Leôncio Diógenes Tavares Câmara 3 , João Monnerat Araújo Ribeiro de Almeida 1 , Emerson Schwingel Ribeiro 2 , Gabriel Francisco da Silva 4 , Lisiane Santos Freitas 5 , Donato Alexandre Gomes Aranda 1
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-08-02 , DOI: 10.1002/ceat.201900609 Samia Tássia Andrade Maciel 1 , Alexander Andrey Lopes da Silva 2 , Yasmin Guimarães Pedro 1 , Cristiano Nunes da Silva 2 , Leôncio Diógenes Tavares Câmara 3 , João Monnerat Araújo Ribeiro de Almeida 1 , Emerson Schwingel Ribeiro 2 , Gabriel Francisco da Silva 4 , Lisiane Santos Freitas 5 , Donato Alexandre Gomes Aranda 1
Affiliation
|
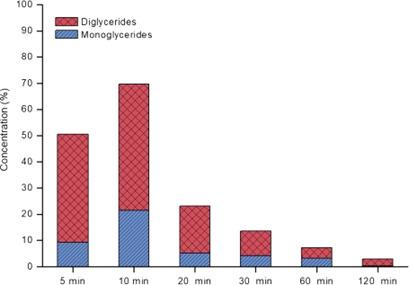
The catalytic hydrolysis of soybean oil was used as an alternative for the production of monoglycerides (MG) and diglycerides (DG). The reactions were conducted in a stainless‐steel tubular reactor in the temperature range of 240–290 °C, on niobium phosphate (NBP) and niobium oxide (NBO) as catalysts. In the hydrolysis reactions at 270 °C, the maximum selectivities of the products of interest were obtained at 22 % MG and 48 % DG for the reaction with NBP, and 7 % MG and 33 % DG with NBO, for 59 % and 36 % of triglyceride conversion in 10 min, respectively. The proposed kinetic model presented a good fit of the theoretical model with the experimental data, showing that the previous hypotheses considered for the mechanism development are suitable for describing the kinetics of soybean oil hydrolysis.
中文翻译:

铌催化剂上大豆油水解动力学
大豆油的催化水解被用作生产甘油单酸酯(MG)和甘油二酸酯(DG)的替代方法。反应在温度为240-290°C的不锈钢管式反应器中,以磷酸铌(NBP)和氧化铌(NBO)为催化剂进行。在270°C的水解反应中,与NBP反应的目标产物的最大选择性为22%MG和48%DG,与NBO反应的为7%MG和33%DG,分别为59%和36%分别在10分钟内的甘油三酸酯转化率。所提出的动力学模型与实验数据很好地吻合了理论模型,表明对于机理发展考虑的先前假设适用于描述大豆油水解的动力学。
更新日期:2020-08-02
中文翻译:

铌催化剂上大豆油水解动力学
大豆油的催化水解被用作生产甘油单酸酯(MG)和甘油二酸酯(DG)的替代方法。反应在温度为240-290°C的不锈钢管式反应器中,以磷酸铌(NBP)和氧化铌(NBO)为催化剂进行。在270°C的水解反应中,与NBP反应的目标产物的最大选择性为22%MG和48%DG,与NBO反应的为7%MG和33%DG,分别为59%和36%分别在10分钟内的甘油三酸酯转化率。所提出的动力学模型与实验数据很好地吻合了理论模型,表明对于机理发展考虑的先前假设适用于描述大豆油水解的动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号