Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2020-08-02 , DOI: 10.1016/j.jece.2020.104336 Nicolás González-Ipia , Kevin Camilo Bolaños-Chamorro , Jawer David Acuña-Bedoya , Fiderman Machuca-Martínez , Samir Fernando Castilla-Acevedo
|
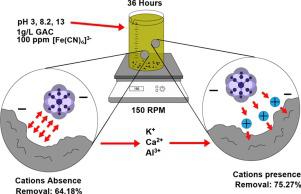
The influence of the addition of cations on the adsorption of [Fe(CN)6]3- on granular activated carbon (GAC) was evaluated. The tests were performed at three pH values (3, 8.2, and 13) to determine the repulsion or electrostatic affinity between the adsorbent and adsorbate. Afterward, the cations (K+, Ca2+, and Al3+) at three pollutant-cation molar ratios (1:1, 1:10, and 1:50) were added to the system, and the influence of those was identified by the changes in the adsorption efficiency. The results show that the higher removal (%) was obtained at pH 13 without neither the presence of iron precipitates nor the liberation of HCN. The adsorption of [Fe(CN)6]3- was enhanced by the addition of K+ at 1:10 and 1:50 molar ratio since higher removals were achieved (75.27% and 76.81% respectively) than those obtained in the absence of cations (64.18%) or in the presence of Ca2+ (67.58%) and Al3+ (65.13%) at a 1:10 molar ratio. The behavior in adsorption in the presence of cations shows that the ion-pair adsorption mechanism can describe the physical phenomenon, showing an increase in the fraction removed and the rate of adsorption with increasing cation charge. The adsorption kinetics using K+ with a 1:10 pollutant-cation molar ratio was fitted to the Lagergren pseudo-first-order model. The GAC adsorption capacity describes the pollutant adsorption rate with the predominance of physical interactions. The experimental data were fitted by the Langmuir and Freundlich isotherms, indicating a monolayer adsorption phenomenon consistent with the previously proposed ion-pair adsorption mechanism.
中文翻译:

通过添加阳离子增强颗粒状活性炭上六氰合铁酸根(III)离子的吸附:在采矿废水处理中的应用
评估了阳离子添加对[Fe(CN)6 ] 3-在颗粒状活性炭(GAC)上的吸附的影响。在三个pH值(3、8.2和13)下进行测试,以确定吸附剂和被吸附物之间的排斥力或静电亲和力。然后,将三种污染物阳离子摩尔比(1:1、1:10和1:50)的阳离子(K +,Ca 2+和Al 3+)添加到系统中,这些因素的影响是通过吸附效率的变化确定。结果表明,在既不存在铁沉淀也不释放HCN的条件下,在pH 13下获得了更高的去除率(%)。[Fe(CN)6 ] 3-的吸附通过在摩尔比为1:10和1:50的条件下添加K +可以提高去除率,因为与不存在阳离子(64.18%)或存在Ca 2的情况相比,去除率更高(分别为75.27%和76.81%)。摩尔比为+(67.58%)和Al 3+(65.13%)。在阳离子存在下的吸附行为表明,离子对吸附机理可以描述物理现象,随着阳离子电荷的增加,去除率和吸附速率都有所提高。使用K +的吸附动力学以1:10的污染物阳离子摩尔比对Lagergren拟一阶模型进行拟合。GAC的吸附能力描述了污染物吸附速率以及物理相互作用的优势。Langmuir和Freundlich等温线拟合了实验数据,表明单层吸附现象与先前提出的离子对吸附机理一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号