Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-08-03 , DOI: 10.1016/j.jsb.2020.107596 Barbara Franke 1 , Marta Veses-Garcia 2 , Kay Diederichs 1 , Heather Allison 2 , Daniel J Rigden 2 , Olga Mayans 1
|
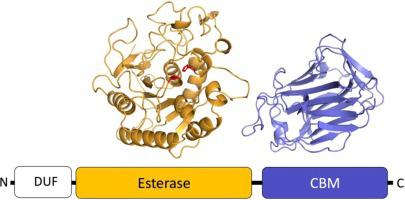
Shiga toxin-encoding bacteriophages transfer Shiga toxin genes to Escherichia coli and are responsible for the emergence of pathogenic bacterial strains that cause severe foodborne human diseases. Gene vb_24B_21 is the most highly conserved gene across sequenced Shiga bacteriophages. Protein vb_24B_21 (also termed 933Wp42 and NanS-p) is a carbohydrate esterase with homology to the E. coli chromosomally encoded NanS that deacetylates sialic acid in the intestinal mucus. To assist the functional characterization of vb_24B_21, we have studied its molecular structure by homology modelling its esterase domain and by elucidating the crystal structure of its uncharacterized C-terminal domain at the atomic resolution of 0.97 Å. Our modelling confirms that NanS from the E. coli host is the closest structurally characterized homolog to the esterase domain of vb_24B_21. Like NanS, vb_24B_21 has an atypical active site, comprising a simple catalytic dyad Ser-His and a divergent oxyanion hole. The crystal structure of the C-terminal domain reveals a lectin-like, jelly-roll β-sandwich fold. The domain displays a prominent cleft that bioinformatics analysis predicts to be a carbohydrate binding site without catalytic properties. In summary, our study indicates that vb_24B_21 is a NanS-like atypical esterase that is assisted by a carbohydrate-binding module of yet undetermined binding specificity.
中文翻译:

编码志贺毒素噬菌体 Φ24B 的保守碳水化合物酯酶 vb_24B_21 的结构注释。
编码志贺毒素的噬菌体将志贺毒素基因转移到大肠杆菌中,并导致致病性细菌菌株的出现,从而导致严重的食源性人类疾病。基因vb_24B_21是已测序志贺菌噬菌体中最高度保守的基因。蛋白质 vb_24B_21(也称为 933Wp42 和 NanS-p)是一种碳水化合物酯酶,与大肠杆菌具有同源性染色体编码的NanS使肠粘液中的唾液酸脱乙酰化。为了协助 vb_24B_21 的功能表征,我们通过对其酯酶结构域的同源性建模和通过以 0.97 Å 的原子分辨率阐明其未表征的 C 端结构域的晶体结构来研究其分子结构。我们的模型证实了来自大肠杆菌的NanS宿主是与 vb_24B_21 的酯酶结构域最接近的结构特征同源物。像 NanS 一样,vb_24B_21 有一个非典型的活性位点,包括一个简单的催化二元组 Ser-His 和一个发散的氧阴离子孔。C 端结构域的晶体结构显示出凝集素样的果冻卷 β 夹心折叠。该域显示一个突出的裂缝,生物信息学分析预测该裂缝是一个没有催化特性的碳水化合物结合位点。总之,我们的研究表明 vb_24B_21 是一种类似 NanS 的非典型酯酶,由尚未确定的结合特异性的碳水化合物结合模块辅助。









































 京公网安备 11010802027423号
京公网安备 11010802027423号