Catalysis Today ( IF 5.2 ) Pub Date : 2020-08-02 , DOI: 10.1016/j.cattod.2020.06.085 Qian Liu 1 , Ce Bian 1 , Yifan Jin 1 , Lei Pang 2 , Zhen Chen 1 , Tao Li 1
|
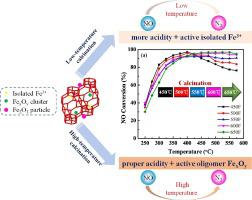
To shed light on the relationships among catalyst calcination temperature, physicochemical properties and catalytic properties of Fe-SSZ-13, a series of catalysts prepared by aqueous post-synthetic exchange and calcined at different temperatures are characterized and evaluated in NH3-SCR reaction. The characterizations and kinetic analysis indicate that the calcination temperature has a great effect on the acidity, Fe species distribution and catalytic properties of the Fe-SSZ-13 catalyst. The rise of calcination temperature decreases the low-temperature activity (< 425℃) and conversely improves the high-temperature activity of Fe-SSZ-13. It could be attributed to the following reasons: under low calcination temperatures, more isolated Fe3+ ions are maintained which can behave as Lewis acid sites and active sites in low reaction temperatures. Simultaneously, stronger surface acidity helps to alleviate NH3 inhibition effect at low temperatures, resulting in good low-temperature activity. On the other hand, the high calcination temperature brings about slight aggregation of isolated Fe3+ to form dimeric/oligomeric Fe clusters, leading more superior high-temperature activity. On the whole, the major active Fe species as well as the contribution of acid sites for low/high temperature activity are different. It could be concluded that the isolated Fe3+ ions and oligomeric Fe clusters are the active sites for low/high SCR reaction temperature, respectively. And zeolite acidity plays a more important role in low-temperature SCR reaction catalyzed by Fe-SSZ-13.
中文翻译:

煅烧温度对 Fe-SSZ-13 催化剂上用于 NO 的 NH3-SCR 的 Fe 物种演化的影响
为了阐明催化剂煅烧温度、物理化学性质和Fe-SSZ-13催化性能之间的关系,对一系列通过水合成后交换制备并在不同温度下煅烧的催化剂在NH 3 -SCR反应中进行了表征和评价。表征和动力学分析表明,煅烧温度对Fe-SSZ-13催化剂的酸度、Fe物种分布和催化性能有很大影响。煅烧温度的升高降低了Fe-SSZ-13的低温活性(<425℃),反之提高了Fe-SSZ-13的高温活性。可归因于以下原因:在较低的煅烧温度下,更多的Fe 3+在低反应温度下可作为路易斯酸位点和活性位点的离子得以保留。同时,较强的表面酸性有助于缓解低温下的NH 3抑制作用,从而产生良好的低温活性。另一方面,较高的煅烧温度导致孤立的Fe 3+轻微聚集形成二聚/低聚Fe簇,从而导致更优异的高温活性。总体而言,主要的活性铁物种以及酸位对低温/高温活性的贡献是不同的。可以得出结论,分离的 Fe 3+离子和低聚铁簇分别是低/高 SCR 反应温度的活性位点。而沸石酸度在Fe-SSZ-13催化的低温SCR反应中起更重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号