当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hyaluronic acid optimises therapeutic effects of hydrogen peroxide-induced oxidative stress on breast cancer.
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-08-01 , DOI: 10.1002/jcp.29957 Ardeshir Abbasi 1 , Nafiseh Pakravan 2 , Zuhair Mohammad Hassan 1
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-08-01 , DOI: 10.1002/jcp.29957 Ardeshir Abbasi 1 , Nafiseh Pakravan 2 , Zuhair Mohammad Hassan 1
Affiliation
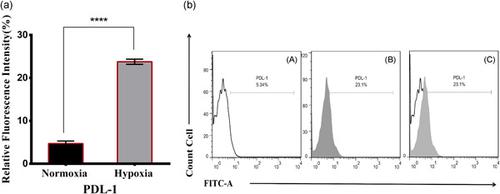
|
Distinguishing the multiple effects of reactive oxygen species (ROS) on cancer cells is important to understand their role in tumour biology. On one side, ROS can be oncogenic by promoting hypoxic conditions, genomic instability and tumorigenesis. Conversely, elevated levels of ROS‐induced oxidative stress can induce cancer cell death. This is evidenced by the conflicting results of research using antioxidant therapy, which in some cases promoted tumour growth and metastasis. However, some antioxidative or ROS‐mediated oxidative therapies have also yielded beneficial effects. To better define the effects of oxidative stress, in vitro experiments were conducted on 4T1 and splenic mononuclear cells (MNCs) under hypoxic and normoxic conditions. Furthermore, hydrogen peroxide (H2O2; 10–1,000 μM) was used as an ROS source alone or in combination with hyaluronic acid (HA), which is frequently used as drug delivery vehicle. Our result indicated that the treatment of cancer cells with H2O2 + HA was significantly more effective than H2O2 alone. In addition, treatment with H2O2 + HA led to increased apoptosis, decreased proliferation, and multiphase cell cycle arrest in 4T1 cells in a dose‐dependent manner under normoxic or hypoxic conditions. As a result, migratory tendency and the messenger RNA levels of vascular endothelial growth factor, matrix metalloproteinase‐2 (MMP‐2), and MMP‐9 were significantly decreased in 4T1 cells. Of note, HA treatment combined with 100–1,000 μM H2O2 caused more damage to MNCs as compared to treatment with lower concentrations (10–50 μM). Based on these results, we propose to administer high‐dose H2O2 + HA (100–1000 μM) for intratumoural injection and low doses for systemic administration. Intratumoural route could have toxic and inhibitory effects not only on the tumour but also on residential myeloid cells defending it, whereas systemic treatment could stimulate peripheral immune responses against the tumour. More in vivo research is required to confirm this hypothesis.
中文翻译:

透明质酸优化过氧化氢诱导的氧化应激对乳腺癌的治疗效果。
区分活性氧 (ROS) 对癌细胞的多重影响对于了解它们在肿瘤生物学中的作用很重要。一方面,ROS 可以通过促进缺氧条件、基因组不稳定性和肿瘤发生而致癌。相反,升高水平的 ROS 诱导的氧化应激可诱导癌细胞死亡。使用抗氧化疗法的研究结果相互矛盾,这证明了这一点,在某些情况下,抗氧化疗法促进了肿瘤的生长和转移。然而,一些抗氧化或 ROS 介导的氧化疗法也产生了有益的效果。为了更好地确定氧化应激的影响,在缺氧和常氧条件下对 4T1 和脾单核细胞 (MNC) 进行了体外实验。此外,过氧化氢(H 2 O 2; 10–1,000 μM) 单独用作 ROS 源或与透明质酸 (HA) 结合使用,透明质酸 (HA) 经常用作药物递送载体。我们的结果表明,肿瘤细胞的用H处理2 Ò 2 + HA是显著不是H更有效2 ö 2独自。此外,用 H 2 O 2 处理 + HA 在常氧或缺氧条件下以剂量依赖性方式导致 4T1 细胞凋亡增加、增殖减少和多相细胞周期停滞。结果,4T1细胞中血管内皮生长因子、基质金属蛋白酶-2(MMP-2)和MMP-9的迁移趋势和信使RNA水平显着降低。值得注意的是,与较低浓度(10–50 μM)的处理相比,HA 处理结合 100–1,000 μM H 2 O 2对 MNC 造成的损害更大。基于这些结果,我们建议给予高剂量 H 2 O 2 + HA (100–1000 μM) 用于瘤内注射和低剂量用于全身给药。瘤内途径不仅对肿瘤而且对保护它的常驻骨髓细胞具有毒性和抑制作用,而全身治疗可以刺激针对肿瘤的外周免疫反应。需要更多的体内研究来证实这一假设。
更新日期:2020-08-01
中文翻译:

透明质酸优化过氧化氢诱导的氧化应激对乳腺癌的治疗效果。
区分活性氧 (ROS) 对癌细胞的多重影响对于了解它们在肿瘤生物学中的作用很重要。一方面,ROS 可以通过促进缺氧条件、基因组不稳定性和肿瘤发生而致癌。相反,升高水平的 ROS 诱导的氧化应激可诱导癌细胞死亡。使用抗氧化疗法的研究结果相互矛盾,这证明了这一点,在某些情况下,抗氧化疗法促进了肿瘤的生长和转移。然而,一些抗氧化或 ROS 介导的氧化疗法也产生了有益的效果。为了更好地确定氧化应激的影响,在缺氧和常氧条件下对 4T1 和脾单核细胞 (MNC) 进行了体外实验。此外,过氧化氢(H 2 O 2; 10–1,000 μM) 单独用作 ROS 源或与透明质酸 (HA) 结合使用,透明质酸 (HA) 经常用作药物递送载体。我们的结果表明,肿瘤细胞的用H处理2 Ò 2 + HA是显著不是H更有效2 ö 2独自。此外,用 H 2 O 2 处理 + HA 在常氧或缺氧条件下以剂量依赖性方式导致 4T1 细胞凋亡增加、增殖减少和多相细胞周期停滞。结果,4T1细胞中血管内皮生长因子、基质金属蛋白酶-2(MMP-2)和MMP-9的迁移趋势和信使RNA水平显着降低。值得注意的是,与较低浓度(10–50 μM)的处理相比,HA 处理结合 100–1,000 μM H 2 O 2对 MNC 造成的损害更大。基于这些结果,我们建议给予高剂量 H 2 O 2 + HA (100–1000 μM) 用于瘤内注射和低剂量用于全身给药。瘤内途径不仅对肿瘤而且对保护它的常驻骨髓细胞具有毒性和抑制作用,而全身治疗可以刺激针对肿瘤的外周免疫反应。需要更多的体内研究来证实这一假设。











































 京公网安备 11010802027423号
京公网安备 11010802027423号