Catalysis Today ( IF 5.2 ) Pub Date : 2020-08-02 , DOI: 10.1016/j.cattod.2020.07.052 João Carlos S. Soares , Arthur Henrique A. Gonçalves , Fátima M.Z. Zotin , Lucia R. Raddi de Araújo , Alexandre B. Gaspar
|
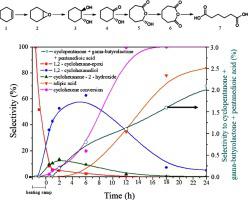
One-step oxidative cleavage of cyclohexene to adipic acid using H2O2 as oxidizing agent and heteropolysalts as heterogeneous catalysts was successfully performed, without the use of additives, such as organic or inorganic acids. The K3PW12O40, K3PMo12O40, Cs3PW12O40 and Cs3PMo12O40 heteropolysalts were synthesized by ion exchange of the respective heteropolyacids using cesium and potassium carbonates. The catalysts were thermally treated at 200 or 600 °C and characterized by Raman spectroscopy, thermogravimetric analysis, X-ray fluorescence (XRF) and photoelectronic X-ray spectroscopy (XPS). Raman results showed that the Keggin structure of the polyoxometalates was successfully obtained for both heteropolyacids and heteropolysalts and remained stable even after thermal treatment at 600 °C. XRF analysis confirmed the insertion of the cations (Cs+ and K+) into the heteropolysalt structures. All catalysts completely converted cyclohexene within 24 h and they were selective to adipic acid. The best catalyst, K3PW12O40 calcined at 600 °C (KPW-600), achieved the highest adipic acid yield (83 %). In this work, we associate the best activity of the KPW-600 heteropolysalt not only to its greater acidity, but also to the higher concentration of (W-O-W)/W species (identified by XPS). Both properties are fundamental for the oxidation reaction of cyclohexene to adipic acid under the experimental conditions used. The results showed that the presence of peroxide species is essential, mainly to favor oxidation reactions, such as the Baeyer-Villiger rearrangement reaction. A reaction scheme for the oxidation of cyclohexene to adipic acid with the best catalyst (KPW-600), was proposed based on our kinetic studies and literature results. In addition to the main route of adipic acid formation from the direct oxidation of cyclohexene, undesirable parallel reactions of the rearrangement of 1,2 - cyclohexanediol and allyl oxidation were also observed. The influence of reaction conditions was also investigated, as well as the reuse of the most promising catalyst.
中文翻译:

反应参数对使用非均相多金属氧酸盐从环己烯合成己二酸的影响
使用H 2 O 2作为氧化剂和杂多盐作为非均相催化剂,成功地进行了环己烯一步氧化裂解为己二酸,而不使用添加剂,例如有机或无机酸。K 3 PW 12 O 40、K 3 PMo 12 O 40、Cs 3 PW 12 O 40和Cs 3 PMo 12 O 40杂多盐是通过使用碳酸铯和碳酸钾对各个杂多酸进行离子交换来合成的。催化剂在 200 或 600 °C 下进行热处理,并通过拉曼光谱、热重分析、X 射线荧光 (XRF) 和光电 X 射线光谱 (XPS) 进行表征。拉曼结果表明,杂多酸和杂多盐均成功获得了多金属氧酸盐的 Keggin 结构,并且即使在 600 °C 热处理后仍保持稳定。XRF 分析证实了阳离子(Cs +和 K +)插入到杂多盐结构中。所有催化剂都在 24 小时内完全转化了环己烯,并且它们对己二酸具有选择性。最好的催化剂,K 3 PW 12Ø 40在 600 °C (KPW-600) 下煅烧,获得了最高的己二酸产率 (83 %)。在这项工作中,我们将 KPW-600 杂多盐的最佳活性不仅与其更高的酸度相关联,而且还与更高浓度的 (WOW)/W 物种(由 XPS 鉴定)相关联。在所用的实验条件下,这两种性质都是环己烯氧化成己二酸的基础。结果表明,过氧化物物质的存在是必不可少的,主要有利于氧化反应,例如拜尔-维利格重排反应。基于我们的动力学研究和文献结果,提出了使用最佳催化剂 (KPW-600) 将环己烯氧化成己二酸的反应方案。除了环己烯直接氧化生成己二酸的主要途径外,还观察到 1,2-环己二醇重排和烯丙基氧化的不希望发生的平行反应。还研究了反应条件的影响,以及最有前途的催化剂的再利用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号