当前位置:
X-MOL 学术
›
Bioeng. Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoparticle‐microglial interaction in the ischemic brain is modulated by injury duration and treatment
Bioengineering & Translational Medicine ( IF 6.1 ) Pub Date : 2020-08-01 , DOI: 10.1002/btm2.10175 Andrea Joseph 1 , Rick Liao 1 , Mengying Zhang 2 , Hawley Helmbrecht 1 , Michael McKenna 1 , Jeremy R Filteau 1 , Elizabeth Nance 1, 2, 3, 4
Bioengineering & Translational Medicine ( IF 6.1 ) Pub Date : 2020-08-01 , DOI: 10.1002/btm2.10175 Andrea Joseph 1 , Rick Liao 1 , Mengying Zhang 2 , Hawley Helmbrecht 1 , Michael McKenna 1 , Jeremy R Filteau 1 , Elizabeth Nance 1, 2, 3, 4
Affiliation
|
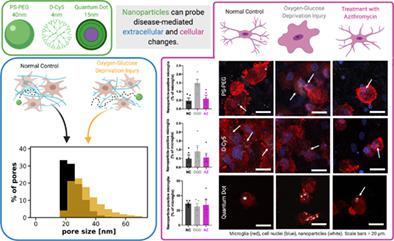
Cerebral ischemia is a major cause of death in both neonates and adults, and currently has no cure. Nanotechnology represents one promising area of therapeutic development for cerebral ischemia due to the ability of nanoparticles to overcome biological barriers in the brain. ex vivo injury models have emerged as a high‐throughput alternative that can recapitulate disease processes and enable nanoscale probing of the brain microenvironment. In this study, we used oxygen–glucose deprivation (OGD) to model ischemic injury and studied nanoparticle interaction with microglia, resident immune cells in the brain that are of increasing interest for therapeutic delivery. By measuring cell death and glutathione production, we evaluated the effect of OGD exposure time and treatment with azithromycin (AZ) on slice health. We found a robust injury response with 0.5 hr of OGD exposure and effective treatment after immediate application of AZ. We observed an OGD‐induced shift in microglial morphology toward increased heterogeneity and circularity, and a decrease in microglial number, which was reversed after treatment. OGD enhanced diffusion of polystyrene‐poly(ethylene glycol) (PS‐PEG) nanoparticles, improving transport and ability to reach target cells. While microglial uptake of dendrimers or quantum dots (QDs) was not enhanced after injury, internalization of PS‐PEG was significantly increased. For PS‐PEG, AZ treatment restored microglial uptake to normal control levels. Our results suggest that different nanoparticle platforms should be carefully screened before application and upon doing so; disease‐mediated changes in the brain microenvironment can be leveraged by nanoscale drug delivery devices for enhanced cell interaction.
中文翻译:

缺血脑中纳米颗粒-小胶质细胞的相互作用受到损伤持续时间和治疗的调节
脑缺血是新生儿和成人死亡的主要原因,目前尚无治愈方法。由于纳米颗粒能够克服大脑中的生物屏障,纳米技术代表了脑缺血治疗发展的一个有前景的领域。离体损伤模型已成为一种高通量替代方案,可以概括疾病过程并能够对大脑微环境进行纳米级探测。在这项研究中,我们使用氧糖剥夺(OGD)来模拟缺血性损伤,并研究了纳米颗粒与小胶质细胞的相互作用,小胶质细胞是大脑中的常驻免疫细胞,对治疗递送越来越感兴趣。通过测量细胞死亡和谷胱甘肽的产生,我们评估了 OGD 暴露时间和阿奇霉素 (AZ) 治疗对切片健康的影响。我们发现 OGD 暴露 0.5 小时后产生了强烈的损伤反应,并且在立即应用 AZ 后进行了有效的治疗。我们观察到 OGD 诱导的小胶质细胞形态发生异质性和圆形性增加的转变,以及小胶质细胞数量的减少,这种变化在治疗后逆转。 OGD 增强了聚苯乙烯-聚乙二醇 (PS-PEG) 纳米粒子的扩散,改善了运输和到达靶细胞的能力。虽然损伤后小胶质细胞对树枝状聚合物或量子点 (QD) 的摄取并未增强,但 PS-PEG 的内化显着增加。对于 PS-PEG,AZ 治疗将小胶质细胞的摄取恢复到正常对照水平。我们的结果表明,不同的纳米颗粒平台应在应用前和应用后仔细筛选;疾病介导的大脑微环境变化可以通过纳米级药物输送装置来增强细胞相互作用。
更新日期:2020-09-23
中文翻译:

缺血脑中纳米颗粒-小胶质细胞的相互作用受到损伤持续时间和治疗的调节
脑缺血是新生儿和成人死亡的主要原因,目前尚无治愈方法。由于纳米颗粒能够克服大脑中的生物屏障,纳米技术代表了脑缺血治疗发展的一个有前景的领域。离体损伤模型已成为一种高通量替代方案,可以概括疾病过程并能够对大脑微环境进行纳米级探测。在这项研究中,我们使用氧糖剥夺(OGD)来模拟缺血性损伤,并研究了纳米颗粒与小胶质细胞的相互作用,小胶质细胞是大脑中的常驻免疫细胞,对治疗递送越来越感兴趣。通过测量细胞死亡和谷胱甘肽的产生,我们评估了 OGD 暴露时间和阿奇霉素 (AZ) 治疗对切片健康的影响。我们发现 OGD 暴露 0.5 小时后产生了强烈的损伤反应,并且在立即应用 AZ 后进行了有效的治疗。我们观察到 OGD 诱导的小胶质细胞形态发生异质性和圆形性增加的转变,以及小胶质细胞数量的减少,这种变化在治疗后逆转。 OGD 增强了聚苯乙烯-聚乙二醇 (PS-PEG) 纳米粒子的扩散,改善了运输和到达靶细胞的能力。虽然损伤后小胶质细胞对树枝状聚合物或量子点 (QD) 的摄取并未增强,但 PS-PEG 的内化显着增加。对于 PS-PEG,AZ 治疗将小胶质细胞的摄取恢复到正常对照水平。我们的结果表明,不同的纳米颗粒平台应在应用前和应用后仔细筛选;疾病介导的大脑微环境变化可以通过纳米级药物输送装置来增强细胞相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号