当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct N‐Alkylation/Fluoroalkylation of Amines Using Carboxylic Acids via Transition‐Metal‐Free Catalysis
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-07-31 , DOI: 10.1002/adsc.202000679 Chunlei Lu 1, 2, 3 , Zetian Qiu 1 , Maojie Xuan 4 , Yan Huang 4 , Yongjia Lou 1 , Yiling Zhu 1 , Hao Shen 1 , Bo‐Lin Lin 1, 2, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-07-31 , DOI: 10.1002/adsc.202000679 Chunlei Lu 1, 2, 3 , Zetian Qiu 1 , Maojie Xuan 4 , Yan Huang 4 , Yongjia Lou 1 , Yiling Zhu 1 , Hao Shen 1 , Bo‐Lin Lin 1, 2, 3
Affiliation
|
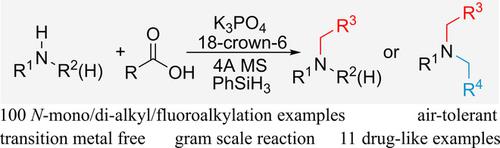
A scalable protocol of direct N‐mono/di‐alkyl/fluoroalkylation of primary/secondary amines has been constructed with various carboxylic acids as coupling agents under the catalysis of a simple air‐tolerant inorganic salt, K3PO4. Advantageous features include 100 examples, 10 drugs and drug‐like amines, fluorinated complex tertiary amines, gram‐scale synthesis and isotope‐labelling amine, thus demonstrating the potential applicability in industry of this methodology. The involvement of relatively less reactive silicon‐hydride compared with the traditional reactive metal‐hydride or boron‐hydride species required to reduce the amide intermediates presumably contributes to the remarkable functional group compatibility.
中文翻译:

通过无过渡金属催化的羧酸直接将胺进行N-烷基/氟烷基化反应
在简单的耐空气无机盐K 3 PO 4的催化下,已使用各种羧酸作为偶联剂构建了伯/仲胺直接N-单/二烷基/氟烷基化的可扩展方案。有利的特征包括100种实例,10种药物和类药物胺,氟化复杂的叔胺,克级合成和同位素标记胺,因此证明了该方法在工业上的潜在适用性。与减少酰胺中间体所需的传统反应性金属氢化物或硼氢化物相比,反应性较小的氢化硅的参与可能有助于显着的官能团相容性。
更新日期:2020-10-06
中文翻译:

通过无过渡金属催化的羧酸直接将胺进行N-烷基/氟烷基化反应
在简单的耐空气无机盐K 3 PO 4的催化下,已使用各种羧酸作为偶联剂构建了伯/仲胺直接N-单/二烷基/氟烷基化的可扩展方案。有利的特征包括100种实例,10种药物和类药物胺,氟化复杂的叔胺,克级合成和同位素标记胺,因此证明了该方法在工业上的潜在适用性。与减少酰胺中间体所需的传统反应性金属氢化物或硼氢化物相比,反应性较小的氢化硅的参与可能有助于显着的官能团相容性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号