当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved detection methods significantly increase the detection window for EPO microdoses.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-07-31 , DOI: 10.1002/dta.2904 Laurent Martin 1 , Jean-Antoine Martin 1 , David Collot 1 , Olivier Hoang 1 , Michel Audran 1 , Magnus Ericsson 1 , Alexandre Marchand 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-07-31 , DOI: 10.1002/dta.2904 Laurent Martin 1 , Jean-Antoine Martin 1 , David Collot 1 , Olivier Hoang 1 , Michel Audran 1 , Magnus Ericsson 1 , Alexandre Marchand 1
Affiliation
|
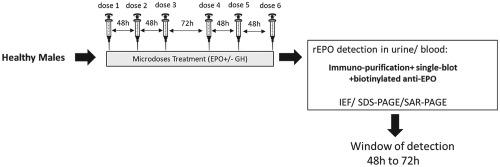
To reproduce a potential doping scenario, a 2 week administration of recombinant erythropoietin (rEPO) microdoses alone or in combination with growth hormone (GH) microdoses (three times a week) was performed on healthy and athletic male subjects. The aim of this study was to evaluate the identification capability of rEPO in samples obtained during and post treatment. Detection was tested in urine and blood using the antidoping techniques for rEPO detection (iso‐electric focusing (IEF)‐, sodium‐dodecyl‐sulfate (SDS)‐polyacrylamide gel electrophoresis (PAGE) and for some urine samples the sarcosyl (SAR)‐PAGE method) with some improvements: for blood samples, instead of a simple concentration step, immuno‐extraction of EPO was performed for all urines to limit protein contamination that can affect migration. In addition, elution buffer modifications also improved the quality of migration. The use of a recently validated biotinylated anti‐EPO antibody simplified the protocols, allowing a single transfer step instead of a double‐blot even by IEF with a lowered background. The criteria for suspicious blood and urine samples by IEF were also re‐evaluated. While endogenous EPO was not decreased over the course of the study, EPO microdoses were detectable in blood and urine between 24 h and 72 h after an administration. Detection in urine in combination with SDS‐PAGE was the most sensitive combination for prolonged detection (100% identification after 48 h, 91% after 72 h), slightly better than IEF. Urine samples also tested by SAR‐PAGE indicated a similar sensitivity of detection to SDS‐PAGE. GH co‐administration had no impact on rEPO elimination/detection.
中文翻译:

改进的检测方法显着增加了 EPO 微剂量的检测窗口。
为了重现潜在的兴奋剂情况,对健康和运动的男性受试者进行了为期 2 周的重组促红细胞生成素 (rEPO) 微剂量单独给药或与生长激素 (GH) 微剂量联合给药(每周 3 次)。本研究的目的是评估 rEPO 在处理期间和处理后获得的样品中的识别能力。使用反兴奋剂技术检测尿液和血液中的 rEPO(等电聚焦 (IEF)-、十二烷基硫酸钠 (SDS)-聚丙烯酰胺凝胶电泳 (PAGE))和一些尿样肌氨酰 (SAR)- PAGE 方法)进行了一些改进:对于血液样本,不是简单的浓缩步骤,而是对所有尿液进行 EPO 的免疫提取,以限制可能影响迁移的蛋白质污染。此外,洗脱缓冲液的修改也提高了迁移质量。使用最近经过验证的生物素化抗 EPO 抗体简化了实验步骤,即使通过具有较低背景的 IEF,也允许单次转移步骤而不是双印迹。还重新评估了 IEF 对可疑血液和尿液样本的标准。虽然在研究过程中内源性 EPO 没有减少,但在给药后 24 至 72 小时之间可在血液和尿液中检测到 EPO 微剂量。尿液中检测结合 SDS-PAGE 是延长检测最灵敏的组合(48 小时后 100% 鉴定,72 小时后 91%),略优于 IEF。还通过 SAR-PAGE 测试的尿液样本表明检测的灵敏度与 SDS-PAGE 相似。GH 联合给药对 rEPO 的消除/检测没有影响。
更新日期:2020-07-31
中文翻译:

改进的检测方法显着增加了 EPO 微剂量的检测窗口。
为了重现潜在的兴奋剂情况,对健康和运动的男性受试者进行了为期 2 周的重组促红细胞生成素 (rEPO) 微剂量单独给药或与生长激素 (GH) 微剂量联合给药(每周 3 次)。本研究的目的是评估 rEPO 在处理期间和处理后获得的样品中的识别能力。使用反兴奋剂技术检测尿液和血液中的 rEPO(等电聚焦 (IEF)-、十二烷基硫酸钠 (SDS)-聚丙烯酰胺凝胶电泳 (PAGE))和一些尿样肌氨酰 (SAR)- PAGE 方法)进行了一些改进:对于血液样本,不是简单的浓缩步骤,而是对所有尿液进行 EPO 的免疫提取,以限制可能影响迁移的蛋白质污染。此外,洗脱缓冲液的修改也提高了迁移质量。使用最近经过验证的生物素化抗 EPO 抗体简化了实验步骤,即使通过具有较低背景的 IEF,也允许单次转移步骤而不是双印迹。还重新评估了 IEF 对可疑血液和尿液样本的标准。虽然在研究过程中内源性 EPO 没有减少,但在给药后 24 至 72 小时之间可在血液和尿液中检测到 EPO 微剂量。尿液中检测结合 SDS-PAGE 是延长检测最灵敏的组合(48 小时后 100% 鉴定,72 小时后 91%),略优于 IEF。还通过 SAR-PAGE 测试的尿液样本表明检测的灵敏度与 SDS-PAGE 相似。GH 联合给药对 rEPO 的消除/检测没有影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号