Journal of Chromatography B ( IF 2.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jchromb.2020.122300 Sumant Saini 1 , Teenu Sharma 1 , Asha Patel 2 , Ranjot Kaur 1 , S K Tripathi 3 , O P Katare 1 , Bhupinder Singh 1
|
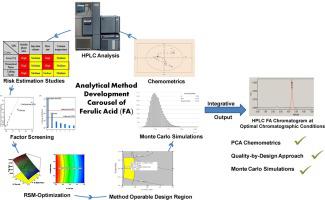
The present work describes the systematic development of a simple, rapid, sensitive, robust, effective and cost-effective reversed-phase high performance liquid chromatographic method for quantitative analysis of ferulic acid using analytical quality by design paradigms. Initially, apt wavelength for the analysis of ferulic acid was selected employing principal component analysis as the chemometric tool. An Ishikawa fishbone diagram was constructed to delineate various plausible variables influencing analytical target profile, viz. peak area, theoretical plate count, retention time and peak tailing as the critical analytical attributes. Risk assessment using risk estimation matrix and factor screening studies employing Taguchi design aided in demarcating two critical method parameters, viz. mobile phase ratio and flow rate affecting critical analytical attributes. Subsequently, the optimum operational conditions of the liquid chromatographic method were delineated using face-centred composite design. Multicollinearity among the chosen factors for optimization was analyzed by the magnitude of variance inflation factor optimized analytical design space, providing optimum method performance, was earmarked using numerical and graphical optimization and corroborated using Monte Carlo simulations. Validation, as per the ICH Q2(R1) guidelines, ratified the efficiency and sensitivity of the developed novel analytical method of ferulic acid in the mobile phase and the human plasma matrix. The optimal method used a mobile phase, comprising of acetonitrile: water (47:53% v/v, pH adjusted to 3.0 with glacial acetic acid), at a flow rate of 0.8 mL·min−1, at a λmax of 322 nm using a C18 column. Use of principal component analysis unearthed the suitable wavelength for analysis, while analytical quality by design approach, along with Monte Carlo simulations, facilitated the identification of influential variables in obtaining the “best plausible” validated chromatographic solution for efficient quantification of ferulic acid.
中文翻译:

QbD指导开发和验证了用于定量阿魏酸的RP-HPLC方法:化学计量工具的合理应用。
本工作描述了一种简单,快速,灵敏,鲁棒,有效且具有成本效益的反相高效液相色谱方法的系统开发,该方法通过设计范式使用分析质量对阿魏酸进行定量分析。最初,使用主成分分析作为化学计量工具选择适合于阿魏酸分析的波长。绘制了石川鱼骨图以描绘影响分析目标轮廓的各种可能变量。峰面积,理论塔板数,保留时间和峰拖尾是关键的分析属性。使用风险估计矩阵进行风险评估,并采用Taguchi设计进行因素筛选研究,以帮助划定两个关键方法参数,即。流动相比率和流速影响关键的分析属性。随后,使用面心复合设计确定了液相色谱方法的最佳操作条件。通过方差膨胀因子优化的分析设计空间的大小分析了所选优化因素之间的多重共线性,从而提供了最佳的方法性能,使用了数值和图形优化来指定用途,并使用蒙特卡洛模拟进行了证实。根据ICH Q2(R1)准则进行的验证,批准了已开发的新型阿魏酸在流动相和人血浆基质中的新型分析方法的效率和灵敏度。最佳方法是使用流动相,流速为0,该流动相由乙腈:水(47:53%v / v,pH用冰醋酸调节至3.0)组成。-1,使用C 18色谱柱在λmax为322 nm处。使用主成分分析法可以找到合适的分析波长,而设计方法的分析质量以及蒙特卡洛模拟方法有助于识别影响变量,从而获得“最佳可行”验证的色谱解决方案,以有效定量阿魏酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号