Catalysis Today ( IF 5.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.cattod.2020.06.083 Alexandra A.P. Mansur 1 , Herman S. Mansur 1 , Sandhra M. Carvalho 1, 2
|
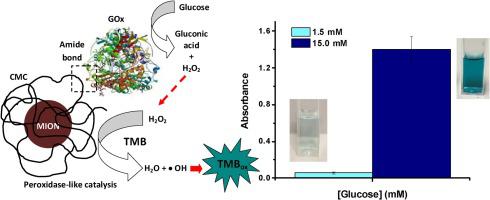
An array of new nanomaterials have been designed to mimic the catalyst characteristics of natural enzymes (termed "nanozymes"), which connects an essential bridge between nanotechnology and biomedical science. Thus, the research on nanozymes has increased dramatically and a new multidisciplinary field has emerged in recent years named nanozymology. Magnetic iron oxide nanoparticles (e.g., Fe2O3 and Fe3O4, MIONs) have revealed to perform an enzyme-like activity with pH-dependent behavior. These nanozymes can catalytically decompose H2O2 into products (e.g., H2O and O2) under mild pH conditions mimicking enzyme-like bioactivity. More importantly, under mildly acidic conditions, they can use H2O2 as the substrate for producing highly toxic reactive oxygen species (ROS) through the generation of hydroxyl radicals ( OH), displaying peroxidase-like activity. Therefore, here we designed and developed a novel class of hybrid nanocatalysts based on the conjugation of GOx (natural enzyme) and MIONs (inorganic nanozyme) stabilized by a biocompatible polymer shell of carboxymethyl cellulose. They created supramolecular vesicle-like nanostructures that were tested for killing brain cancer cells (U-87 MG) in vitro, where they proved anticancer activity attributed to ferroptosis-induced cell death. These hybrid nanostructures performed as a cascade of integrated nanocatalysts: a) GOx worked as the starting catalyst, where the glucose in the medium generated H2O2; b) next, H2O2 was catalyzed by the downstream MION nanozymes (Fe3O4) via Fenton-like reactions releasing toxic species (ROS), which caused the cancer cell death. Remarkably, this nanozyme-induced cell death was more pronounced in brain cancer cells ascribed to the more acidic microenvironment than on healthy cells.
OH), displaying peroxidase-like activity. Therefore, here we designed and developed a novel class of hybrid nanocatalysts based on the conjugation of GOx (natural enzyme) and MIONs (inorganic nanozyme) stabilized by a biocompatible polymer shell of carboxymethyl cellulose. They created supramolecular vesicle-like nanostructures that were tested for killing brain cancer cells (U-87 MG) in vitro, where they proved anticancer activity attributed to ferroptosis-induced cell death. These hybrid nanostructures performed as a cascade of integrated nanocatalysts: a) GOx worked as the starting catalyst, where the glucose in the medium generated H2O2; b) next, H2O2 was catalyzed by the downstream MION nanozymes (Fe3O4) via Fenton-like reactions releasing toxic species (ROS), which caused the cancer cell death. Remarkably, this nanozyme-induced cell death was more pronounced in brain cancer cells ascribed to the more acidic microenvironment than on healthy cells.
中文翻译:

基于多糖-酶-磁性氧化铁纳米结构的工程混合纳米酶催化剂级联在癌症治疗中的潜在应用
已经设计了一系列新的纳米材料来模拟天然酶(称为“纳米酶”)的催化剂特性,它连接了纳米技术和生物医学科学之间的重要桥梁。因此,对纳米酶的研究急剧增加,近年来出现了一个新的多学科领域,称为纳米酶学。磁性氧化铁纳米颗粒(例如, Fe 2 O 3和 Fe 3 O 4,MIONs)已显示出具有pH依赖性行为的酶样活性。这些纳米酶可以将H 2 O 2催化分解成产物(例如H 2 O和O 2) 在模拟酶样生物活性的温和 pH 条件下。更重要的是,在弱酸性条件下,它们可以以H 2 O 2为底物,通过产生羟基自由基(OH)来产生剧毒的活性氧(ROS) ,表现出类过氧化物酶的活性。因此,在这里,我们设计并开发了一类新型混合纳米催化剂,该催化剂基于由羧甲基纤维素的生物相容性聚合物壳稳定的 GOx(天然酶)和 MION(无机纳米酶)的共轭。他们创造了超分子囊泡状纳米结构,经测试可在体外杀死脑癌细胞(U-87 MG),他们证明了归因于铁死亡诱导的细胞死亡的抗癌活性。这些混合纳米结构作为集成纳米催化剂的级联执行: a) GOx 作为起始催化剂,其中介质中的葡萄糖产生 H 2 O 2;b) 接下来,H 2 O 2被下游的 MION 纳米酶 (Fe 3 O 4 )通过类芬顿反应催化释放有毒物质 (ROS),从而导致癌细胞死亡。值得注意的是,与健康细胞相比,这种纳米酶诱导的细胞死亡在脑癌细胞中更为明显,这归因于更酸性的微环境。
,表现出类过氧化物酶的活性。因此,在这里,我们设计并开发了一类新型混合纳米催化剂,该催化剂基于由羧甲基纤维素的生物相容性聚合物壳稳定的 GOx(天然酶)和 MION(无机纳米酶)的共轭。他们创造了超分子囊泡状纳米结构,经测试可在体外杀死脑癌细胞(U-87 MG),他们证明了归因于铁死亡诱导的细胞死亡的抗癌活性。这些混合纳米结构作为集成纳米催化剂的级联执行: a) GOx 作为起始催化剂,其中介质中的葡萄糖产生 H 2 O 2;b) 接下来,H 2 O 2被下游的 MION 纳米酶 (Fe 3 O 4 )通过类芬顿反应催化释放有毒物质 (ROS),从而导致癌细胞死亡。值得注意的是,与健康细胞相比,这种纳米酶诱导的细胞死亡在脑癌细胞中更为明显,这归因于更酸性的微环境。











































 京公网安备 11010802027423号
京公网安备 11010802027423号