Catalysis Today ( IF 5.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.cattod.2020.07.038 Xiaowa Nie , Zeshi Zhang , Haozhi Wang , Xinwen Guo , Chunshan Song
|
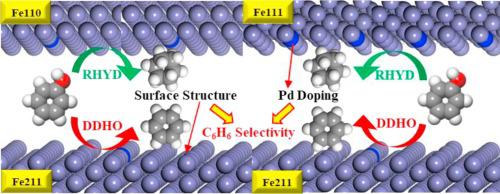
Density functional theory (DFT) calculations were performed to investigate the effects of surface structure and Pd doping of Fe catalysts on the activity and selectivity of phenol hydrodeoxygenation (HDO). Phenol adsorption via binding with the aromatic ring was found to be energetically more stable than adsorbing through solely the hydroxyl group on Fe(110), Fe(111) and Fe(211) surfaces. The adsorption strength on monometallic Fe surfaces increased in the sequence of Fe(211) < Fe(110) < Fe(111). Pd doping did not have a significant influence on the adsorption stability of phenol. The (211) facet of Fe featured particular steps showed good activity toward aromatics production, as evidenced by the lower barriers associated with the dehydroxylation followed by H addition to form benzene than the direct hydrogenation on the aromatic ring. In contrast, the ring saturation products would be dominant on Fe(110) and Fe(111) surfaces due to lower hydrogenation barriers on the aromatic ring of phenol as compared to the CAr-O bond cleavage. The kinetically preferred pathway for phenol conversion on Pd doped Fe(110) and Fe(111) surfaces was the direct hydrogenation on the aromatic ring, same to that identified on monometallic (110) and (111) surfaces. In comparison, on Fe(211), the difference in kinetic barrier between dehydroxylation and ring hydrogenation pathways became larger in the presence of Pd, promoting the selectivity to benzene formation. In the presence of Pd dopant, the H2 molecule was readily dissociated and the surface H* coverage was increased, leading to a lowering of the adsorption enthalpy of key surface species and ultimately enhancing the rates of HDO.
中文翻译:

Fe催化剂的表面结构和Pd掺杂对苯酚选择性加氢脱氧的影响
进行了密度泛函理论(DFT)计算,以研究Fe催化剂的表面结构和Pd掺杂对苯酚加氢脱氧(HDO)活性和选择性的影响。发现通过与芳环结合而吸附的苯酚在能量上比仅通过Fe(110),Fe(111)和Fe(211)表面上的羟基吸附更稳定。在单金属Fe表面上的吸附强度按Fe(211)<Fe(110)<Fe(111)的顺序增加。钯的掺杂对苯酚的吸附稳定性没有显着影响。Fe的(211)刻面具有特定的步骤,对芳烃的生产具有良好的活性,这与脱羟基,随后H加成苯相比,与芳环上的直接氢化相比,具有较低的阻隔性,这证明了这一点。Ar -O键断裂。在Pd掺杂的Fe(110)和Fe(111)表面上进行苯酚转化的动力学首选途径是在芳环上进行直接氢化,与在单金属(110)和(111)表面上确定的氢化相同。相比之下,在Fe(211)上,在Pd的存在下,脱羟基和环氢化途径之间的动力学势垒差异变大,从而促进了对苯形成的选择性。在Pd掺杂剂的存在下,H 2分子易于解离,表面H *覆盖率增加,从而导致关键表面物质的吸附焓降低,最终提高了HDO的速率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号