Biochemical and Biophysical Research Communications ( IF 2.5 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.bbrc.2020.07.070 Yuan He 1 , Jian Kang 1 , Jianxing Song 1
|
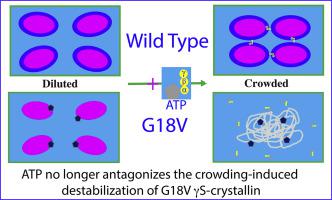
In lens, ∼90% of ocular proteins are αβγ-crystallins with concentrations ≥400 mg/ml, which need to remain soluble for the whole life-span and their aggregation leads to cataract. The G18V mutation of human γS-crystallin causes hereditary childhood-onset cortical cataract. Mysteriously, despite being a metabolically-quiescent organ, lens maintains ATP concentrations of 3–7 mM. Very recently, we found that ATP has no significant binding to γS-crystallin as well as no alternation of its conformation. Nevertheless, ATP antagonizes the crowding-induced destabilization of γS-crystallin even at 1:1, most likely by interacting with the hydration shell. Here by DSF and NMR, we characterized the effect of ATP on binding, conformation, stability of G18V γS-crystallin and its interactions with α-crystallin. The results reveal: 1) G18V significantly accelerates the crowding-induced destabilization with Tm of 67 °C reduced to 50.5 °C at 1 mM. 2) Most unexpectedly, G18V almost completely eliminates the antagonizing effect of ATP against the crowding-induced destabilization. 3) ATP shows no significant effect on the interactions of α-crystallin with both WT and G18V γS-crystallin. Results together decode for the first time that G18V causes cataract not only by accelerating the crowding-induced destabilization, but also by eliminating the antagonizing effect of ATP against the crowding-induced destabilization.
中文翻译:

引起白内障的G18V消除了ATP对抗由人群引起的人γS-晶状蛋白不稳定的拮抗作用。
在晶状体中,约90%的眼部蛋白质是浓度≥400mg / ml的αβγ-晶状体蛋白,它们在整个寿命中都必须保持可溶,并且它们的聚集会导致白内障。人γS-晶状体蛋白的G18V突变导致遗传性的儿童期皮质性白内障。神秘地,尽管是代谢静止的器官,晶状体仍保持3-7 mM的ATP浓度。最近,我们发现ATP与γS-晶状蛋白没有明显的结合,也没有构象的改变。然而,ATP即使在1:1时也能拮抗γS-晶状体蛋白的拥挤诱导的失稳,这很可能是与水合壳相互作用引起的。在这里,通过DSF和NMR,我们表征了ATP对G18VγS-晶状蛋白的结合,构象,稳定性及其与α-晶状蛋白的相互作用的影响。结果显示:1)G18V显着加速了拥挤引起的不稳定,Tm为67°C,在1 mM下降至50.5°C。2)最出乎意料的是,G18V几乎完全消除了ATP对拥挤诱导的不稳定的拮抗作用。3)ATP对α-结晶蛋白与WT和G18VγS-结晶蛋白的相互作用没有显着影响。结果首次共同解释了G18V不仅通过加速拥挤引起的不稳定,而且消除了ATP对拥挤引起的不稳定的拮抗作用而引起白内障。3)ATP对α-结晶蛋白与WT和G18VγS-结晶蛋白的相互作用没有显着影响。结果首次一起解码,表明G18V不仅通过加速拥挤引起的不稳定,而且还消除了ATP对拥挤引起的不稳定的拮抗作用而引起白内障。3)ATP对α-结晶蛋白与WT和G18VγS-结晶蛋白的相互作用没有显着影响。结果首次一起解码,表明G18V不仅通过加速拥挤引起的不稳定,而且还消除了ATP对拥挤引起的不稳定的拮抗作用而引起白内障。









































 京公网安备 11010802027423号
京公网安备 11010802027423号