Advanced Powder Technology ( IF 4.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.apt.2020.07.011 Guixia Fan , Chaofan Zhang , Taojin Wang , Jiushuai Deng , Yijun Cao , Luping Chang , Guoli Zhou , Yankun Wu , Peng Li
|
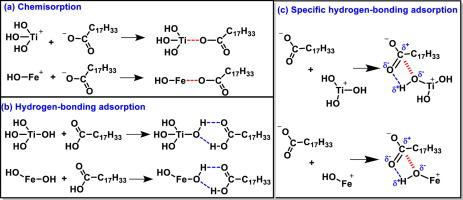
Titanaugite particles are detrimental to the flotation of fine ilmenite. In this work, the surface adsorption thermodynamic, kinetic properties and the adsorption mechanism of sodium oleate on ilmenite and titanaugite surfaces were investigated by measuring surface wettability, lipophilic hydrophilic ratio, surface free energy, adsorption rate, adsorption density and adsorption thickness. The hydrophilicity of the minerals were determined by the contact angle, lipophilic hydrophilic ratio, value, and value. The surface wettability results showed that the hydrophilicity of the minerals decreased following: −20 μm titanaugite >−20 μm ilmenite >−38 + 20 μm ilmenite >− 74 + 38 μm ilmenite. The adsorption process can be represented with Langmuir isotherm and a pseudo second-order kinetic model. Calculation of the adsorption thermodynamic parameters confirmed that the adsorption of sodium oleate on ilmenite surfaces is spontaneous, feasible, and endothermic. And it was alsoconfirmed that sodium oleate was adsorbed onto ilmenite due to both physical and chemical adsorption. In situ AFM imaging visually demonstrated that the adsorption density and thickness of the sodium oleate adsorbed onto titanaugite were lower than those of sodium oleate on ilmenite, which was consistent with the adsorption thermodynamic parameters. It was further confirmed that the electrostatic interaction, chemisorption, hydrogen-bonding adsorption and specific hydrogen-bonding adsorption were the main mechanisms for the sodium oleate adsorbed onto ilmenite, as the zeta potential and XPS spectra analyses.
中文翻译:

油酸钠在钛铁矿和钛铁矿上的表面吸附热力学,动力学性质和吸附机理的新见解
钛金矿颗粒不利于细钛铁矿的浮选。在这项工作中,通过测量表面润湿性,亲脂性亲水比,表面自由能,吸附速率,吸附密度和吸附厚度,研究了钛酸钠和钛铁矿表面油酸钠的表面吸附热力学,动力学性质和吸附机理。矿物的亲水性由接触角,亲脂性亲水比, 值,以及 值。表面润湿性结果表明,矿物的亲水性降低如下:-20μm钛铁矿> -20μm钛铁矿> -38 + 20μm钛铁矿>-74 + 38μm钛铁矿。吸附过程可以用Langmuir等温线和伪二级动力学模型表示。吸附热力学参数的计算证实,油酸钠在钛铁矿表面上的吸附是自发的,可行的并且是吸热的。并且还确认了由于物理和化学吸附,油酸钠被吸附在钛铁矿上。原位原子力显微镜成像结果表明,钛铁矿上吸附的油酸钠的吸附密度和厚度均小于钛铁矿上的油酸钠的吸附密度和厚度,这与吸附热力学参数一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号