当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Emissive tetraphenylethylene (TPE) derivatives in a dissolved state tightly fastened by a short oligo(ethylene glycol) chain
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0qo00839g Yuma Tanaka 1, 2, 3, 4 , Takashi Machida 1, 2, 3, 4 , Toshiaki Noumi 2, 3, 4, 5, 6 , Kazuki Sada 1, 2, 3, 4, 5 , Kenta Kokado 2, 3, 4, 7, 8
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0qo00839g Yuma Tanaka 1, 2, 3, 4 , Takashi Machida 1, 2, 3, 4 , Toshiaki Noumi 2, 3, 4, 5, 6 , Kazuki Sada 1, 2, 3, 4, 5 , Kenta Kokado 2, 3, 4, 7, 8
Affiliation
|
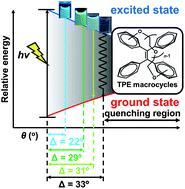
The structural change in the excited state evidently plays a crucial role in the quenching process of organic molecules exhibiting aggregation-induced emission (AIE, thus AIEgens) in the solution state. In this report, we synthesized a series of tetraphenylethylene (TPE) macrocycles having a covalent oligoethylene glycol (OEG) linkage between vicinal phenyl rings with various chain lengths and substituting positions. As a result, the obtained TPE macrocycles which are tightly fastened by short OEG chains showed strong emission even in the solution state. The tight fastener efficiently restricted the π twist around the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in TPE macrocycles, which was also supported by theoretical computations. These results provide very important information about the origin of the AIE properties of TPE derivatives, which will lead to the rational design of new AIEgens.
C bond in TPE macrocycles, which was also supported by theoretical computations. These results provide very important information about the origin of the AIE properties of TPE derivatives, which will lead to the rational design of new AIEgens.
中文翻译:

溶解状态的发光四苯乙烯(TPE)衍生物通过短寡(乙二醇)链紧密固定
激发态的结构变化显然在溶液状态下表现出聚集诱导发射(AIE,因此称为AIEgens)的有机分子的猝灭过程中起着至关重要的作用。在这份报告中,我们合成了一系列四苯基乙烯(TPE)大环,它们在具有不同链长和取代位置的邻位苯环之间具有共价低聚乙二醇(OEG)键。结果,即使在溶液状态下,通过短OEG链紧密固定的所得TPE大环也显示出强发射。紧密的紧固件有效地限制了中心C周围的π扭曲![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) TPE大环中的C键也得到理论计算的支持。这些结果提供了有关TPE衍生物AIE特性起源的非常重要的信息,这将导致新AIEgens的合理设计。
TPE大环中的C键也得到理论计算的支持。这些结果提供了有关TPE衍生物AIE特性起源的非常重要的信息,这将导致新AIEgens的合理设计。
更新日期:2020-09-16
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in TPE macrocycles, which was also supported by theoretical computations. These results provide very important information about the origin of the AIE properties of TPE derivatives, which will lead to the rational design of new AIEgens.
C bond in TPE macrocycles, which was also supported by theoretical computations. These results provide very important information about the origin of the AIE properties of TPE derivatives, which will lead to the rational design of new AIEgens.
中文翻译:

溶解状态的发光四苯乙烯(TPE)衍生物通过短寡(乙二醇)链紧密固定
激发态的结构变化显然在溶液状态下表现出聚集诱导发射(AIE,因此称为AIEgens)的有机分子的猝灭过程中起着至关重要的作用。在这份报告中,我们合成了一系列四苯基乙烯(TPE)大环,它们在具有不同链长和取代位置的邻位苯环之间具有共价低聚乙二醇(OEG)键。结果,即使在溶液状态下,通过短OEG链紧密固定的所得TPE大环也显示出强发射。紧密的紧固件有效地限制了中心C周围的π扭曲
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) TPE大环中的C键也得到理论计算的支持。这些结果提供了有关TPE衍生物AIE特性起源的非常重要的信息,这将导致新AIEgens的合理设计。
TPE大环中的C键也得到理论计算的支持。这些结果提供了有关TPE衍生物AIE特性起源的非常重要的信息,这将导致新AIEgens的合理设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号