当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A holistic view of mammalian (vertebrate) cellular iron uptake.
Metallomics ( IF 2.9 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0mt00065e Daniel J Kosman 1
Metallomics ( IF 2.9 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0mt00065e Daniel J Kosman 1
Affiliation
|
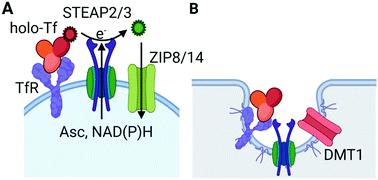
Cell iron uptake in mammals is commonly distinguished by whether the iron is presented to the cell as transferrin-bound or not: TBI or NTBI. This generic perspective conflates TBI with canonical transferrin receptor, endosomal iron uptake, and NTBI with uptake supported by a plasma membrane-localized divalent metal ion transporter, most often identified as DMT1. In fact, iron uptake by mammalian cells is far more nuanced than this somewhat proscribed view suggests. This view fails to accommodate the substantial role that ZIP8 and ZIP14 play in iron uptake, while adhering to the traditional premise that a relatively high endosomal [H+] is thermodynamically required for release of iron from holo-Tf. The canonical view of iron uptake also does not encompass the fact that plasma membrane electron transport – PMET – has long been linked to cell iron uptake. In fact, the known mammalian metallo-reductases – Dcytb and the STEAP proteins – are members of this cohort of cytochrome-dependent oxido-reductases that shuttle reducing equivalents across the plasma membrane. A not commonly appreciated fact is the reduction potential of ferric iron in holo-Tf is accessible to cytoplasmic reducing equivalents – reduced pyridine and flavin mono- and di-nucleotides and dihydroascorbic acid. This allows for the reductive release of Fe2+ at the extracellular surface of the PM and subsequent transport into the cytoplasm by a neutral pH transporter – a ZIP protein. What this perspective emphasizes is that there are two TfR-dependent uptake pathways, one which does and one which does not involve clathrin-dependent, endolysosomal trafficking. This raises the question as to the selective advantage of having two Tf, TfR-dependent routes of iron accumulation. This review of canonical and non-canonical iron uptake uses cerebral iron trafficking as a point of discussion, a focus that encourages inclusion also of the importance of ferritin as a circulating ‘chaperone’ of ferric iron.
中文翻译:

哺乳动物(脊椎动物)细胞铁摄取的整体视图。
哺乳动物的细胞铁摄取通常通过铁是否以转铁蛋白结合的形式呈现给细胞:TBI 或 NTBI。这种通用观点将 TBI 与经典转铁蛋白受体、内体铁摄取和 NTBI 与质膜定位的二价金属离子转运蛋白支持的摄取混为一谈,通常被确定为 DMT1。事实上,哺乳动物细胞对铁的吸收比这种有点被禁止的观点所暗示的要微妙得多。这种观点未能适应 ZIP8 和 ZIP14 在铁吸收中发挥的重要作用,同时坚持传统前提,即从全息释放铁在热力学上需要相对较高的内体 [H+]。-Tf。铁摄取的规范观点也不包括质膜电子传递(PMET)长期以来与细胞铁摄取有关的事实。事实上,已知的哺乳动物金属还原酶——Dcytb 和 STEAP 蛋白——是这组细胞色素依赖性氧化还原酶的成员,它们将还原当量穿梭在质膜上。甲不常用理解的事实是三价铁在还原电位全息-Tf是细胞质的还原当量访问-还原的吡啶和黄素单和二核苷酸和二氢抗坏血酸。这允许 Fe 2+的还原释放在 PM 的细胞外表面,随后通过中性 pH 转运蛋白(ZIP 蛋白)转运到细胞质中。这个观点强调的是有两种 TfR 依赖的摄取途径,一种涉及网格蛋白依赖的内溶酶体运输,另一种不涉及。这提出了关于具有两条 Tf、TfR 依赖性铁积累途径的选择性优势的问题。这篇关于经典和非经典铁摄取的综述使用大脑铁运输作为讨论点,这一焦点鼓励将铁蛋白作为三价铁循环“伴侣”的重要性也纳入其中。
更新日期:2020-09-23
中文翻译:

哺乳动物(脊椎动物)细胞铁摄取的整体视图。
哺乳动物的细胞铁摄取通常通过铁是否以转铁蛋白结合的形式呈现给细胞:TBI 或 NTBI。这种通用观点将 TBI 与经典转铁蛋白受体、内体铁摄取和 NTBI 与质膜定位的二价金属离子转运蛋白支持的摄取混为一谈,通常被确定为 DMT1。事实上,哺乳动物细胞对铁的吸收比这种有点被禁止的观点所暗示的要微妙得多。这种观点未能适应 ZIP8 和 ZIP14 在铁吸收中发挥的重要作用,同时坚持传统前提,即从全息释放铁在热力学上需要相对较高的内体 [H+]。-Tf。铁摄取的规范观点也不包括质膜电子传递(PMET)长期以来与细胞铁摄取有关的事实。事实上,已知的哺乳动物金属还原酶——Dcytb 和 STEAP 蛋白——是这组细胞色素依赖性氧化还原酶的成员,它们将还原当量穿梭在质膜上。甲不常用理解的事实是三价铁在还原电位全息-Tf是细胞质的还原当量访问-还原的吡啶和黄素单和二核苷酸和二氢抗坏血酸。这允许 Fe 2+的还原释放在 PM 的细胞外表面,随后通过中性 pH 转运蛋白(ZIP 蛋白)转运到细胞质中。这个观点强调的是有两种 TfR 依赖的摄取途径,一种涉及网格蛋白依赖的内溶酶体运输,另一种不涉及。这提出了关于具有两条 Tf、TfR 依赖性铁积累途径的选择性优势的问题。这篇关于经典和非经典铁摄取的综述使用大脑铁运输作为讨论点,这一焦点鼓励将铁蛋白作为三价铁循环“伴侣”的重要性也纳入其中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号