当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ag2Cu2O3 – a catalyst template material for selective electroreduction of CO to C2+ products
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-07-30 , DOI: 10.1039/d0ee01100b Nemanja Martić 1, 2, 3, 4 , Christian Reller 1, 2, 3, 4 , Chandra Macauley 3, 4, 5, 6 , Mario Löffler 3, 4, 7, 8, 9 , Andreas M. Reichert 3, 4, 7, 8, 9 , Thomas Reichbauer 1, 2, 3, 4 , Kim-Marie Vetter 1, 2, 3, 4 , Bernhard Schmid 1, 2, 3, 4 , David McLaughlin 3, 4, 7, 8, 9 , Paul Leidinger 4, 10, 11, 12 , David Reinisch 1, 2, 3, 4 , Christoph Vogl 1, 2, 3, 4 , Karl J. J. Mayrhofer 3, 4, 7, 8, 9 , Ioannis Katsounaros 3, 4, 7, 8 , Günter Schmid 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-07-30 , DOI: 10.1039/d0ee01100b Nemanja Martić 1, 2, 3, 4 , Christian Reller 1, 2, 3, 4 , Chandra Macauley 3, 4, 5, 6 , Mario Löffler 3, 4, 7, 8, 9 , Andreas M. Reichert 3, 4, 7, 8, 9 , Thomas Reichbauer 1, 2, 3, 4 , Kim-Marie Vetter 1, 2, 3, 4 , Bernhard Schmid 1, 2, 3, 4 , David McLaughlin 3, 4, 7, 8, 9 , Paul Leidinger 4, 10, 11, 12 , David Reinisch 1, 2, 3, 4 , Christoph Vogl 1, 2, 3, 4 , Karl J. J. Mayrhofer 3, 4, 7, 8, 9 , Ioannis Katsounaros 3, 4, 7, 8 , Günter Schmid 1, 2, 3, 4
Affiliation
|
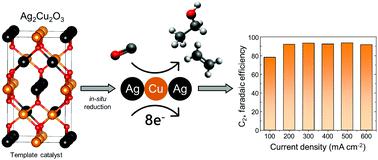
Although recent years have brought significant progress within the field of electrochemical conversion of CO2 and CO to value-added chemicals, many more challenges need to be overcome for this technology to be implemented on an industrial level. Rational design of catalyst materials that would enable selective production of desired products at industrially relevant current densities (>200 mA cm−2) is most certainly one of them. Here, we introduce Ag2Cu2O3, a mixed-metal oxide, as a starting template material for efficient electroreduction of CO to C2+ products. By combining results from electrochemical real-time mass spectrometry (EC-RTMS), XRD and XPS we confirmed the template nature of Ag2Cu2O3 and in situ formation of a fully reduced CuAg bimetallic material during the first minutes of electrolysis. Electrochemical screening of the catalyst revealed significantly varying product distributions when CO2 (CO2RR) and CO (CORR) where used as feed gases. During CORR, a faradaic efficiency close to 92% towards C2+ products at 600 mA cm−2 was achieved. On the other hand, during CO2RR, CO was found to be the main product under all tested current densities, reaching a maximum faradaic efficiency of 68%. XPS valence band spectra of the bimetallic surface originating from Ag2Cu2O3 showed that its d-band electronic structure is noticeably different compared to metallic Ag and Cu, a finding we link to the observed product distributions. Finally, additional microscopy characterization techniques were used to investigate the observed surface reconstruction of the catalyst material under reaction conditions.
中文翻译:

Ag2Cu2O3 –用于将CO选择性电还原为C2 +产品的催化剂模板材料
尽管近年来在将CO 2和CO电化学转化为增值化学品方面取得了重大进展,但是要在工业水平上实施该技术,还需要克服许多挑战。能够以工业相关电流密度(> 200 mA cm -2)选择性生产所需产品的催化剂材料的合理设计无疑是其中之一。在这里,我们介绍一种混合金属氧化物Ag 2 Cu 2 O 3作为有效将CO有效还原为C 2+的起始模板材料。产品。通过结合电化学实时质谱(EC-RTMS),XRD和XPS的结果,我们确认了Ag 2 Cu 2 O 3的模板性质以及在电解的第一分钟内原位形成完全还原的CuAg双金属材料。当将CO 2(CO 2 RR)和CO(CORR)用作进料气时,催化剂的电化学筛选显示出明显不同的产物分布。在CORR期间,对于600 mA cm -2的C 2+产品,其法拉第效率接近92%。另一方面,在CO 2期间发现在所有测试的电流密度下,RR,CO是主要产品,最大法拉第效率达到68%。源自Ag 2 Cu 2 O 3的双金属表面的XPS价带谱表明,其d带电子结构与金属Ag和Cu相比明显不同,这一发现与观察到的产物分布有关。最后,使用额外的显微镜表征技术来研究在反应条件下观察到的催化剂材料的表面重构。
更新日期:2020-09-16
中文翻译:

Ag2Cu2O3 –用于将CO选择性电还原为C2 +产品的催化剂模板材料
尽管近年来在将CO 2和CO电化学转化为增值化学品方面取得了重大进展,但是要在工业水平上实施该技术,还需要克服许多挑战。能够以工业相关电流密度(> 200 mA cm -2)选择性生产所需产品的催化剂材料的合理设计无疑是其中之一。在这里,我们介绍一种混合金属氧化物Ag 2 Cu 2 O 3作为有效将CO有效还原为C 2+的起始模板材料。产品。通过结合电化学实时质谱(EC-RTMS),XRD和XPS的结果,我们确认了Ag 2 Cu 2 O 3的模板性质以及在电解的第一分钟内原位形成完全还原的CuAg双金属材料。当将CO 2(CO 2 RR)和CO(CORR)用作进料气时,催化剂的电化学筛选显示出明显不同的产物分布。在CORR期间,对于600 mA cm -2的C 2+产品,其法拉第效率接近92%。另一方面,在CO 2期间发现在所有测试的电流密度下,RR,CO是主要产品,最大法拉第效率达到68%。源自Ag 2 Cu 2 O 3的双金属表面的XPS价带谱表明,其d带电子结构与金属Ag和Cu相比明显不同,这一发现与观察到的产物分布有关。最后,使用额外的显微镜表征技术来研究在反应条件下观察到的催化剂材料的表面重构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号