当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics and kinetics of protonated merocyanine photoacids in water
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0sc03152f Cesare Berton 1 , Daniel Maria Busiello 2 , Stefano Zamuner 2 , Euro Solari 1 , Rosario Scopelliti 1 , Farzaneh Fadaei-Tirani 1 , Kay Severin 1 , Cristian Pezzato 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0sc03152f Cesare Berton 1 , Daniel Maria Busiello 2 , Stefano Zamuner 2 , Euro Solari 1 , Rosario Scopelliti 1 , Farzaneh Fadaei-Tirani 1 , Kay Severin 1 , Cristian Pezzato 1
Affiliation
|
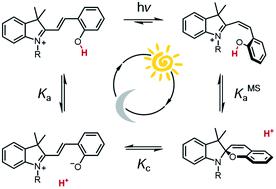
Metastable-state photoacids (mPAHs) are chemical species whose photo-activated state is long-lived enough to allow for proton diffusion. Liao's photoacid (1) represents the archetype of mPAHs, and is being widely used on account of its unique capability to change the acidity of aqueous solutions reversibly. The behavior of 1 in water, however, still remains poorly understood. Herein, we provide in-depth insights on the thermodynamics and kinetics of 1 in water through a series of comparative 1H NMR and UV-Vis studies and relative modelling. Under dark conditions, we quantified a three-component equilibrium system where the dissociation (Ka) of the open protonated form (MCH) is followed by isomerization (Kc) of the open deprotonated form (MC) to the closed spiropyran form (SP) – i.e., in the absence of light, the ground state acidity can be expressed as KGSa = Ka(1 + Kc). On the other hand, under powerful and continuous light irradiation we were able to assess, for the first time experimentally, the dissociation constant (KMSa) of the protonated metastable state (cis-MCH). In addition, we found that thermal ring-opening of SP is always rate-determining regardless of pH, whereas hydrolysis is reminiscent of what is found for Schiff bases. The proposed methodology is general, and it was applied to two other compounds bearing a shorter (ethyl, 2) and a longer (butyl, 3) alkyl-1-sulfonate bridge. We found that the pKa remains constant, whereas both pKc and pKMSa linearly increase with the length of the alkyl bridge. Importantly, all results are consistent with a four-component model cycle, which describes perfectly the full dynamics of proton release/uptake of 1–3 in water. The superior hydrolytic stability and water solubility of compound 3, together with its relatively high pKGSa (low Kc), allowed us to achieve fully reversible jumps of 2.5 pH units over 18 consecutive cycles (6 hours).
中文翻译:

水中质子化部花青光酸的热力学和动力学
亚稳态光酸 (mPAH) 是一种化学物质,其光激活态寿命足够长,足以允许质子扩散。廖氏光酸( 1 )代表了mPAHs的原型,由于其独特的可逆改变水溶液酸度的能力而被广泛应用。然而, 1在水中的行为仍然知之甚少。在此,我们通过一系列1 H NMR 和 UV-Vis 比较研究以及相关建模,深入了解1在水中的热力学和动力学。在黑暗条件下,我们量化了一个三组分平衡系统,其中开放质子化形式(MCH)的解离( Ka )随后是开放去质子化形式(MC)异构化( Kc )到封闭螺吡喃形式(SP ) ) –即在没有光的情况下,基态酸度可表示为K GS a = Ka (1 + K c )。另一方面,在强而连续的光照射下,我们首次通过实验评估了质子化亚稳态( cis -MCH)的解离常数( K MS a )。此外,我们发现,无论 pH 值如何,SP 的热开环始终是速率决定因素,而水解则让人想起席夫碱的情况。 所提出的方法是通用的,并且它适用于另外两种带有较短(乙基, 2 )和较长(丁基, 3 )烷基-1-磺酸酯桥的化合物。我们发现p K a保持恒定,而p K c和p K MS a都随着烷基桥的长度线性增加。重要的是,所有结果都与四组分模型循环一致,该模型完美地描述了水中质子释放/吸收1-3的完整动力学。化合物3具有优异的水解稳定性和水溶性,以及相对较高的 p K GS a (低K c ),使我们能够在 18 个连续循环(6 小时)内实现 2.5 pH 单位的完全可逆跳跃。
更新日期:2020-08-20
中文翻译:

水中质子化部花青光酸的热力学和动力学
亚稳态光酸 (mPAH) 是一种化学物质,其光激活态寿命足够长,足以允许质子扩散。廖氏光酸( 1 )代表了mPAHs的原型,由于其独特的可逆改变水溶液酸度的能力而被广泛应用。然而, 1在水中的行为仍然知之甚少。在此,我们通过一系列1 H NMR 和 UV-Vis 比较研究以及相关建模,深入了解1在水中的热力学和动力学。在黑暗条件下,我们量化了一个三组分平衡系统,其中开放质子化形式(MCH)的解离( Ka )随后是开放去质子化形式(MC)异构化( Kc )到封闭螺吡喃形式(SP ) ) –即在没有光的情况下,基态酸度可表示为K GS a = Ka (1 + K c )。另一方面,在强而连续的光照射下,我们首次通过实验评估了质子化亚稳态( cis -MCH)的解离常数( K MS a )。此外,我们发现,无论 pH 值如何,SP 的热开环始终是速率决定因素,而水解则让人想起席夫碱的情况。 所提出的方法是通用的,并且它适用于另外两种带有较短(乙基, 2 )和较长(丁基, 3 )烷基-1-磺酸酯桥的化合物。我们发现p K a保持恒定,而p K c和p K MS a都随着烷基桥的长度线性增加。重要的是,所有结果都与四组分模型循环一致,该模型完美地描述了水中质子释放/吸收1-3的完整动力学。化合物3具有优异的水解稳定性和水溶性,以及相对较高的 p K GS a (低K c ),使我们能够在 18 个连续循环(6 小时)内实现 2.5 pH 单位的完全可逆跳跃。











































 京公网安备 11010802027423号
京公网安备 11010802027423号