当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of an Efficient Scale-Up Synthesis Method for a β3-Adrenergic Receptor Agonist, Ritobegron Ethyl Hydrochloride
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acs.oprd.0c00278 Junichi Sonehara 1, 2 , Hidetoshi Isawa 3 , Kiyoshi Kasai 3 , Norihiko Kikuchi 3 , Takehiro Ishikawa 4 , Masahiro Kobayashi 3 , Tetsuji Ozawa 3 , Ritsu Suzuki 2 , Makoto Kobayashi 2 , Takayuki Ainai 2 , Masayuki Nishimura 2 , Yohei Saito 1 , Kyoko Nakagawa-Goto 1, 5
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acs.oprd.0c00278 Junichi Sonehara 1, 2 , Hidetoshi Isawa 3 , Kiyoshi Kasai 3 , Norihiko Kikuchi 3 , Takehiro Ishikawa 4 , Masahiro Kobayashi 3 , Tetsuji Ozawa 3 , Ritsu Suzuki 2 , Makoto Kobayashi 2 , Takayuki Ainai 2 , Masayuki Nishimura 2 , Yohei Saito 1 , Kyoko Nakagawa-Goto 1, 5
Affiliation
|
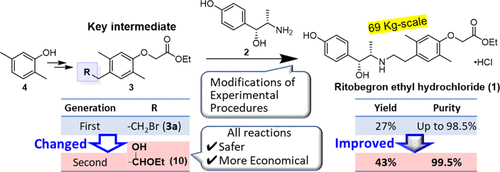
An efficient route for the multikilogram synthesis of a selective β3-adrenergic receptor agonist, ritobegron ethyl hydrochloride (1), was developed by changing the coupling method of 4-hydroxynorephedrine (2) with phenoxyacetate 3. This new method successfully overcame several obstacles in the first-generation method via the use of aldehyde 3b derived from hemiacetal 10 to couple with 2. The main advantages of the key intermediate 10 were that it was sufficiently stable to enable handling at large scales, it could be synthesized without an exothermic reaction or excessive use of expensive reagents, and it had the ability to reduce impurities by purification prior to coupling with 2. The resulting second-generation method improved the overall yield from 27 to 43% and the purity from up to 98.5 to 99.5%. Furthermore, it was effective for 69 kg-scale synthesis, representing a major improvement over several hundreds grams scale of the first-generation method.
中文翻译:

对于β的高效大规模合成方法的建立3肾上腺素受体激动剂,Ritobegron盐酸甲酸乙酯
用于选择性β的数千克合成的高效路线3 -肾上腺素能受体激动剂,ritobegron乙基盐酸盐(1),通过改变4- hydroxynorephedrine(的耦合方法开发2与苯氧基)3。通过使用半缩醛10衍生的醛3b与2偶联,该新方法成功克服了第一代方法中的几个障碍。关键中间体10的主要优点它具有足够的稳定性,可以进行大规模处理,可以合成而无需放热反应或过量使用昂贵的试剂,并且具有在与2偶联之前通过纯化减少杂质的能力。所得的第二代方法将总收率从27%提高至43%,并将纯度提高了98.5至99.5%。此外,它对69千克规模的合成有效,代表了对第一代方法几百克规模的重大改进。
更新日期:2020-09-20
中文翻译:

对于β的高效大规模合成方法的建立3肾上腺素受体激动剂,Ritobegron盐酸甲酸乙酯
用于选择性β的数千克合成的高效路线3 -肾上腺素能受体激动剂,ritobegron乙基盐酸盐(1),通过改变4- hydroxynorephedrine(的耦合方法开发2与苯氧基)3。通过使用半缩醛10衍生的醛3b与2偶联,该新方法成功克服了第一代方法中的几个障碍。关键中间体10的主要优点它具有足够的稳定性,可以进行大规模处理,可以合成而无需放热反应或过量使用昂贵的试剂,并且具有在与2偶联之前通过纯化减少杂质的能力。所得的第二代方法将总收率从27%提高至43%,并将纯度提高了98.5至99.5%。此外,它对69千克规模的合成有效,代表了对第一代方法几百克规模的重大改进。











































 京公网安备 11010802027423号
京公网安备 11010802027423号