当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Commercial Manufacturing Route to 2-Fluoroadenine, The Key Unnatural Nucleobase of Islatravir
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acs.oprd.0c00304 Cynthia M. Hong 1 , Yingju Xu 1 , John Y. L. Chung 1 , Danielle M. Schultz 1 , Mark Weisel 1 , Richard J. Varsolona 1 , Yong-Li Zhong 1 , Akasha K. Purohit 1 , Cyndi Q. He 1 , Donald R. Gauthier 1 , Guy R. Humphrey 1 , Kevin M. Maloney 1 , François Lévesque 1 , Zhixun Wang 1 , Aaron M. Whittaker 1 , Eric Sirota 1 , Jonathan P. McMullen 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-31 , DOI: 10.1021/acs.oprd.0c00304 Cynthia M. Hong 1 , Yingju Xu 1 , John Y. L. Chung 1 , Danielle M. Schultz 1 , Mark Weisel 1 , Richard J. Varsolona 1 , Yong-Li Zhong 1 , Akasha K. Purohit 1 , Cyndi Q. He 1 , Donald R. Gauthier 1 , Guy R. Humphrey 1 , Kevin M. Maloney 1 , François Lévesque 1 , Zhixun Wang 1 , Aaron M. Whittaker 1 , Eric Sirota 1 , Jonathan P. McMullen 1
Affiliation
|
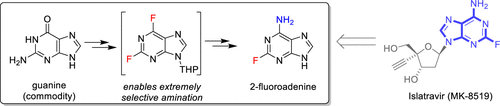
We report the practical synthesis of a key fragment of islatravir (MK-8591), a novel nucleoside reverse transcriptase translocation inhibitor (NRTTI) currently under investigation for treatment and pre-exposure prophylaxis (PrEP) against HIV infection. The fragment, the unnatural nucleobase 2-fluoroadenine, is incorporated into MK-8591 via a biocatalytic aldol-glycosylation cascade, which imposes stringent requirements for its synthesis and isolation. Presented herein is the development work leading to a practical, scalable route from guanine, featuring a dual fluorination approach to a novel 9-THP-2,6-difluoropurine intermediate that enables a mild, highly selective, direct amination. This one-pot fluorination/amination sequence utilizes a direct isolation to deliver high purity 9-THP-2-fluoroadenine, which features ideal properties with respect to reactivity, solubility, and crystallinity. An acid-catalyzed liberation of 2-fluoroadenine in aqueous buffer delivers the appropriate purity profile to facilitate the enzymatic cascade to access MK-8591.
中文翻译:

开发一条商业化生产路线,以2-氟丁烯酸为基础,这是Islatravir的关键非天然核碱基
我们报告了islatravir(MK-8591),一种新型的核苷逆转录酶易位抑制剂(NRTTI)的关键片段的实际合成,该抑制剂目前正在研究中,针对HIV感染的治疗和暴露前预防(PrEP)。该片段(非天然核碱基2-氟腺嘌呤)通过生物催化的羟醛糖基化级联反应并入MK-8591,这对其合成和分离提出了严格的要求。本文介绍的是开发从鸟嘌呤到实用,可扩展路线的开发工作,其特征在于采用双氟化方法开发新颖的9-THP-2,6-二氟嘌呤中间体,从而实现温和,高度选择性的直接胺化。这种一锅式氟化/胺化步骤利用直接分离来提供高纯度的9-THP-2-氟腺嘌呤,在反应性,溶解性和结晶度方面具有理想的特性。在水性缓冲液中酸催化的2-氟腺嘌呤释放可提供适当的纯度,以利于酶联级联反应进入MK-8591。
更新日期:2020-07-31
中文翻译:

开发一条商业化生产路线,以2-氟丁烯酸为基础,这是Islatravir的关键非天然核碱基
我们报告了islatravir(MK-8591),一种新型的核苷逆转录酶易位抑制剂(NRTTI)的关键片段的实际合成,该抑制剂目前正在研究中,针对HIV感染的治疗和暴露前预防(PrEP)。该片段(非天然核碱基2-氟腺嘌呤)通过生物催化的羟醛糖基化级联反应并入MK-8591,这对其合成和分离提出了严格的要求。本文介绍的是开发从鸟嘌呤到实用,可扩展路线的开发工作,其特征在于采用双氟化方法开发新颖的9-THP-2,6-二氟嘌呤中间体,从而实现温和,高度选择性的直接胺化。这种一锅式氟化/胺化步骤利用直接分离来提供高纯度的9-THP-2-氟腺嘌呤,在反应性,溶解性和结晶度方面具有理想的特性。在水性缓冲液中酸催化的2-氟腺嘌呤释放可提供适当的纯度,以利于酶联级联反应进入MK-8591。











































 京公网安备 11010802027423号
京公网安备 11010802027423号