当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of SUMO Binding Proteins Enriched after Covalent Photo-Cross-Linking.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-07-31 , DOI: 10.1021/acschembio.0c00609 Kira Brüninghoff , Annika Aust , Kim F. Taupitz , Stephanie Wulff , Wolfgang Dörner , Henning D. Mootz
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-07-31 , DOI: 10.1021/acschembio.0c00609 Kira Brüninghoff , Annika Aust , Kim F. Taupitz , Stephanie Wulff , Wolfgang Dörner , Henning D. Mootz
|
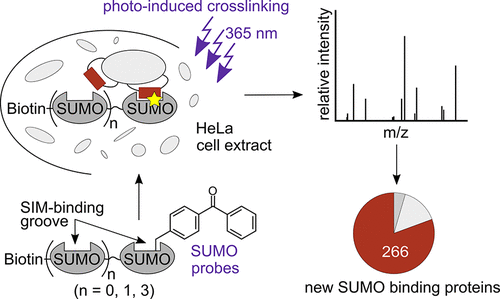
Post-translational modification with the small ubiquitin-like modifier (SUMO) affects thousands of proteins in the human proteome and is implicated in numerous cellular processes. The main outcome of SUMO conjugation is a rewiring of protein–protein interactions through recognition of the modifier’s surface by SUMO binding proteins. The SUMO-interacting motif (SIM) mediates binding to a groove on SUMO; however, the low affinity of this interaction and the poor conservation of SIM sequences complicates the isolation and identification of SIM proteins. To address these challenges, we have designed and biochemically characterized monomeric and multimeric SUMO-2 probes with a genetically encoded photo-cross-linker positioned next to the SIM binding groove. Following photoinduced covalent capture, even weak SUMO binders are not washed away during the enrichment procedure, and very stringent washing conditions can be applied to remove nonspecifically binding proteins. A total of 329 proteins were isolated from nuclear HeLa cell extracts and identified using mass spectrometry. We found the molecular design of our probes was corroborated by the presence of many established SUMO interacting proteins and the high percentage (>90%) of hits containing a potential SIM sequence, as predicted by bioinformatic analyses. Notably, 266 of the 329 proteins have not been previously reported as SUMO binders using traditional noncovalent enrichment procedures. We confirmed SUMO binding with purified proteins and mapped the position of the covalent cross-links for selected cases. We postulate a new SIM in MRE11, involved in DNA repair. The identified SUMO binding candidates will help to reveal the complex SUMO-mediated protein network.
中文翻译:

共价光交联后富集的SUMO结合蛋白的鉴定。
用小泛素样修饰子(SUMO)进行的翻译后修饰会影响人类蛋白质组中的数千种蛋白质,并涉及许多细胞过程。SUMO缀合的主要结果是通过SUMO结合蛋白识别修饰物表面来重新连接蛋白质间相互作用。SUMO交互基序(SIM)介导与SUMO上凹槽的结合;然而,这种相互作用的亲和力低和SIM序列的保守性差使SIM蛋白的分离和鉴定复杂化。为了解决这些挑战,我们设计并通过生物化学方法对单体和多聚体SUMO-2探针进行了生物化学表征,其探针具有遗传编码的光交联剂,紧邻SIM结合槽。在光诱导的共价捕获之后,即使是薄弱的SUMO结合剂,在富集过程中也不会被洗掉,非常严格的洗涤条件可用于去除非特异性结合的蛋白。从核HeLa细胞提取物中分离出总共329种蛋白质,并使用质谱进行了鉴定。我们发现,探针的分子设计得到了许多已建立的SUMO相互作用蛋白的证实,并且如生物信息学分析所预测的那样,命中率高(> 90%)的命中含有潜在的SIM序列。值得注意的是,以前没有使用传统的非共价富集程序将329种蛋白质中的266种报道为SUMO结合物。我们确认了SUMO与纯化蛋白的结合,并确定了所选病例的共价交联位置。我们在MRE11中假设了一个新的SIM,该DNA修复涉及到。
更新日期:2020-09-20
中文翻译:

共价光交联后富集的SUMO结合蛋白的鉴定。
用小泛素样修饰子(SUMO)进行的翻译后修饰会影响人类蛋白质组中的数千种蛋白质,并涉及许多细胞过程。SUMO缀合的主要结果是通过SUMO结合蛋白识别修饰物表面来重新连接蛋白质间相互作用。SUMO交互基序(SIM)介导与SUMO上凹槽的结合;然而,这种相互作用的亲和力低和SIM序列的保守性差使SIM蛋白的分离和鉴定复杂化。为了解决这些挑战,我们设计并通过生物化学方法对单体和多聚体SUMO-2探针进行了生物化学表征,其探针具有遗传编码的光交联剂,紧邻SIM结合槽。在光诱导的共价捕获之后,即使是薄弱的SUMO结合剂,在富集过程中也不会被洗掉,非常严格的洗涤条件可用于去除非特异性结合的蛋白。从核HeLa细胞提取物中分离出总共329种蛋白质,并使用质谱进行了鉴定。我们发现,探针的分子设计得到了许多已建立的SUMO相互作用蛋白的证实,并且如生物信息学分析所预测的那样,命中率高(> 90%)的命中含有潜在的SIM序列。值得注意的是,以前没有使用传统的非共价富集程序将329种蛋白质中的266种报道为SUMO结合物。我们确认了SUMO与纯化蛋白的结合,并确定了所选病例的共价交联位置。我们在MRE11中假设了一个新的SIM,该DNA修复涉及到。











































 京公网安备 11010802027423号
京公网安备 11010802027423号