Plant Physiology ( IF 6.5 ) Pub Date : 2020-10-01 , DOI: 10.1104/pp.20.00461 Anushobha Regmi 1 , Jay Shockey 2 , Hari Kiran Kotapati 3 , Philip D Bates 3, 4
|
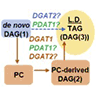
Seed triacylglycerol (TAG) biosynthesis involves a metabolic network containing multiple different diacylglycerol (DAG) and acyl donor substrate pools. This network of pathways overlaps with those for essential membrane lipid synthesis and utilizes multiple different classes of TAG biosynthetic enzymes. Acyl flux through this network ultimately dictates the final oil fatty acid composition. Most strategies to alter seed oil composition involve the overexpression of lipid biosynthetic enzymes, but how these enzymes are assembled into metabolons and which substrate pools are used by each is still not well understood. To understand the roles of different classes of TAG biosynthetic acyltransferases in seed oil biosynthesis, we utilized the Arabidopsis (Arabidopsis thaliana) diacylglycerol acyltransferase mutant dgat1-1 (in which phosphatidylcholine:diacylglycerol acyltransferase (AtPDAT1) is the major TAG biosynthetic enzyme), and enhanced TAG biosynthesis by expression of Arabidopsis acyltransferases AtDGAT1 and AtDGAT2, as well as the DGAT2 enzymes from soybean (Glycine max), and castor (Ricinus communis), followed by isotopic tracing of glycerol flux through the lipid metabolic network in developing seeds. The results indicate each acyltransferase has a unique effect on seed oil composition. AtDGAT1 produces TAG from a rapidly produced phosphatidylcholine-derived DAG pool. However, AtPDAT1 and plant DGAT2 enzymes utilize a different and larger bulk phosphatidylcholine-derived DAG pool that is more slowly turned over for TAG biosynthesis. Based on metabolic fluxes and protein:protein interactions, our model of TAG synthesis suggests that substrate channeling to select enzymes and spatial separation of different acyltransferases into separate metabolons affect efficient TAG production and oil fatty acid composition.
中文翻译:

含有 DGAT1 的产油代谢物利用与含有 DGAT2 或 PDAT 的产油代谢物不同的底物池。
种子三酰甘油(TAG)生物合成涉及包含多种不同二酰甘油(DAG)和酰基供体底物池的代谢网络。该途径网络与必需的膜脂合成途径重叠,并利用多种不同类别的 TAG 生物合成酶。通过该网络的酰基通量最终决定了最终的油脂肪酸组成。大多数改变种子油成分的策略涉及脂质生物合成酶的过度表达,但这些酶如何组装成代谢物以及每种酶使用哪些底物库仍不清楚。为了了解不同类别的 TAG 生物合成酰基转移酶在种子油生物合成中的作用,我们利用拟南芥 ( Arabidopsis thaliana ) 二酰基甘油酰基转移酶突变体dgat1-1 (其中磷脂酰胆碱:二酰基甘油酰基转移酶 (AtPDAT1) 是主要的 TAG 生物合成酶),并增强通过表达拟南芥酰基转移酶 AtDGAT1 和 AtDGAT2 以及来自大豆 ( Glycine max ) 和蓖麻 ( Ricinus communis ) 的 DGAT2 酶进行 TAG 生物合成,然后通过发育种子中脂质代谢网络的甘油通量进行同位素追踪。结果表明每种酰基转移酶对种子油成分具有独特的影响。 AtDGAT1 从快速产生的磷脂酰胆碱衍生的 DAG 池中产生 TAG。然而,AtPDAT1 和植物 DGAT2 酶利用不同且更大的大量磷脂酰胆碱衍生 DAG 库,该库用于 TAG 生物合成的速度更慢。 基于代谢通量和蛋白质:蛋白质相互作用,我们的 TAG 合成模型表明,选择酶的底物通道和不同酰基转移酶在空间上分离成单独的代谢物会影响有效的 TAG 生产和油脂肪酸组成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号