当前位置:
X-MOL 学术
›
J. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced step‐growth polymerization of thieno[3,4‐b] thiophene derivatives. The substitution effect on the reactivity and electrochemical properties
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2020-07-30 , DOI: 10.1002/pol.20200398 Tugba Celiker 1 , Recep İsci 1 , Kerem Kaya 1 , Turan Ozturk 1, 2 , Yusuf Yagci 1
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2020-07-30 , DOI: 10.1002/pol.20200398 Tugba Celiker 1 , Recep İsci 1 , Kerem Kaya 1 , Turan Ozturk 1, 2 , Yusuf Yagci 1
Affiliation
|
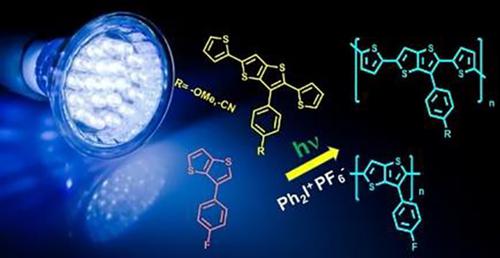
We herein report photoinduced step‐growth polymerization of highly conjugated 2,5‐dithiophenyl (thieno[3,4‐b] thiophene) (TTs) derivatives possessing 4‐cyanophenyl or 4‐methoxyphenyl or 3‐(4‐fluorophenyl) groups substituted at the terminal position. Upon irradiation at 350 nm, the excited state of these molecules forms exciplex with diphenyliodonium hexafluorophosphate (DPI) that undergoes electron transfer reaction forming radical cations. Successive proton release and radical coupling reactions yield corresponding oligothienothiophenes with overall yields varying between 19–74%. Structural and molecular weight characteristics of the oligomers thus formed were investigated by Ultraviolet, fluorescence, NMR (Nuclear Magnetic Resonance) and infrared (IR) spectroscopic methods, and GPC (Gel Permeation Chromatography), respectively. The effect of substitution and dithiophene side groups on the reactivity of the monomer and band gap of the oligomers formed was evaluated by using cyclic voltammetry.
中文翻译:

噻吩并[3,4-b]噻吩衍生物的光诱导逐步增长聚合。取代对反应活性和电化学性能的影响
我们在此报告了高度共轭的2,5-二噻吩(噻吩并[3,4-b]噻吩)(TTs)衍生物的光诱导逐步增长聚合反应,该衍生物具有在以下位置取代的4-氰基苯基或4-甲氧基苯基或3-(4-氟苯基)基团终端位置。在350 nm照射后,这些分子的激发态与六氟磷酸二苯基碘鎓(DPI)形成激基复合物,后者经历电子转移反应形成自由基阳离子。连续的质子释放和自由基偶联反应产生相应的寡硫噻吩,总产率在19-74%之间。分别通过紫外线,荧光,NMR(核磁共振)和红外(IR)光谱法以及GPC(凝胶渗透色谱法)研究由此形成的低聚物的结构和分子量特征。
更新日期:2020-07-30
中文翻译:

噻吩并[3,4-b]噻吩衍生物的光诱导逐步增长聚合。取代对反应活性和电化学性能的影响
我们在此报告了高度共轭的2,5-二噻吩(噻吩并[3,4-b]噻吩)(TTs)衍生物的光诱导逐步增长聚合反应,该衍生物具有在以下位置取代的4-氰基苯基或4-甲氧基苯基或3-(4-氟苯基)基团终端位置。在350 nm照射后,这些分子的激发态与六氟磷酸二苯基碘鎓(DPI)形成激基复合物,后者经历电子转移反应形成自由基阳离子。连续的质子释放和自由基偶联反应产生相应的寡硫噻吩,总产率在19-74%之间。分别通过紫外线,荧光,NMR(核磁共振)和红外(IR)光谱法以及GPC(凝胶渗透色谱法)研究由此形成的低聚物的结构和分子量特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号