Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-07-31 , DOI: 10.1016/j.jmb.2020.07.018 Yi Wen 1 , Gerald W Feigenson 1 , Volker M Vogt 1 , Robert A Dick 1
|
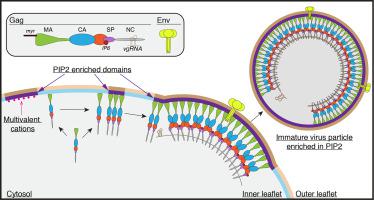
Phosphatidylinositol 4,5-bisphosphate (PIP2) is critical for HIV-1 virus assembly. The viral membrane is enriched in PIP2, suggesting that the virus assembles at PIP2-rich microdomains. We showed previously that in model membranes PIP2 can form nanoscopic clusters bridged by multivalent cations. Here, using purified proteins we quantitated the binding of HIV-1 Gag-related proteins to giant unilamellar vesicles containing either clustered or free PIP2. Myristoylated MA strongly preferred binding to clustered PIP2. By contrast, unmyristoylated HIV-1 MA, RSV MA, and a PH domain all preferred to interact with free PIP2. We also found that HIV-1 Gag multimerization promotes PIP2 clustering. Truncated Gag proteins comprising the MA, CA, and SP domains (MACASP) or the MA and CA domains (MACA) induced self-quenching of acyl chain-labeled fluorescent PIP2 in liposomes, implying clustering. However, HIV-1 MA itself did not induce PIP2 clustering. A CA inter-hexamer dimer interface mutation led to a loss of induced PIP2 clustering in MACA, indicating the importance of protein multimerization. Cryo-electron tomography of liposomes with bound MACA showed an amorphous protein layer on the membrane surface. Thus, it appears that while protein–protein interactions are required for PIP2 clustering, formation of a regular lattice is not. Protein-induced PIP2 clustering and multivalent cation-induced PIP2 clustering are additive. Taken together, these results provide the first evidence that HIV-1 Gag can selectively target pre-existing PIP2-enriched domains of the plasma membrane for viral assembly, and that Gag multimerization can further enrich PIP2 at assembly sites. These effects could explain the observed PIP2 enrichment in HIV-1.
中文翻译:

HIV-1病毒膜中PI(4,5)P2富集的机制。
磷脂酰肌醇4,5-二磷酸(PIP2)对于HIV-1病毒的组装至关重要。病毒膜富含PIP2,表明该病毒在富含PIP2的微域聚集。先前我们表明,在模型膜中,PIP2可以形成由多价阳离子桥接的纳米簇。在这里,我们使用纯化的蛋白定量了HIV-1 Gag相关蛋白与含有簇状或游离PIP2的巨大单层囊泡的结合。肉豆蔻酰化的MA强烈优选结合成簇的PIP2。相比之下,未豆蔻酰化的HIV-1 MA,RSV MA和PH结构域都倾向于与游离PIP2相互作用。我们还发现,HIV-1 Gag多聚化可促进PIP2聚类。截短的Gag蛋白,包含MA,CA,和SP域(MACASP)或MA和CA域(MACA)诱导脂质体中酰基链标记的荧光PIP2的自猝灭,暗示聚簇。但是,HIV-1 MA本身不会诱导PIP2聚集。CA间六聚体间二聚体界面突变导致MACA中诱导的PIP2簇丢失,表明蛋白质多聚化的重要性。结合了MACA的脂质体的低温电子层析成像显示,膜表面上存在无定形蛋白质层。因此,似乎蛋白质间相互作用是PIP2聚簇所必需的,但规则的晶格却不是必需的。蛋白质诱导的PIP2簇和多价阳离子诱导的PIP2簇是可加的。在一起 这些结果提供了第一个证据,证明HIV-1 Gag可以选择性地靶向预先存在的PIP2富集的质膜域进行病毒组装,并且Gag的多聚化可以进一步富集组装部位的PIP2。这些影响可以解释观察到的PIP2在HIV-1中的富集。











































 京公网安备 11010802027423号
京公网安备 11010802027423号