Catalysis Communications ( IF 3.4 ) Pub Date : 2020-07-31 , DOI: 10.1016/j.catcom.2020.106125 Rodrigo Webber , Muhammad I. Qadir , Eduardo Sola , Marta Martín , Elizabeth Suárez , Jairton Dupont
|
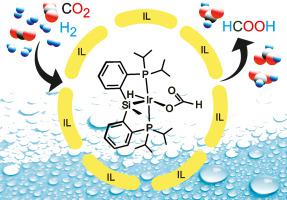
Complex [IrClH{κP,P,Si-Si(Me)(C6H4–2-PiPr2)2}] (1) showed a remarkable catalytic activity for CO2 hydrogenation in a DMSO/H2O solvent system incorporating 1,2-dimethyl-3-butylimidazolium acetate ionic liquid (IL), producing 0.94 M formic acid with initial TOFs up to 1432 h−1 (CO2/H2 = 20/40 bar, 30 °C). While the hydrogenation outcome followed dependences upon gas composition, pressure and temperature similar to those of other efficient systems in DMSO/H2O, the kinetic dependence upon catalyst loading revealed non-linear effects suggestive of relevant IL-catalyst interactions. NMR speciation studies identified two major complexes, [Ir(OCHO)(H){κP,P,Si-Si(Me)(C6H4–2-PiPr2)2}] (2) and [Ir(H)2{κP,P,Si-Si(Me)(C6H4–2-PiPr2)2}(DMSO)] (3), potentially responsible for catalytic cycling though inactive outside the current solvent system.
中文翻译:

在DMSO /水/离子液体溶剂系统中,Ir(PSiP)夹钳氢化物将CO 2快速氢化为甲酸
络合物[IrClH {κP,P,Si-Si(Me)(C 6 H 4 –2-P i Pr 2)2 }](1)在DMSO / H 2 O溶剂中显示出显着的CO 2加氢催化活性。该系统包含乙酸1,2-二甲基-3-丁基咪唑鎓离子液体(IL),可生产0.94 M甲酸,其初始TOF最高可达1432 h -1(CO 2 / H 2 = 20/40 bar,30°C)。氢化结果取决于气体成分,压力和温度,与DMSO / H 2中其他有效系统的结果相似O,对催化剂负载量的动力学依赖性揭示了暗示相关的IL-催化剂相互作用的非线性效应。NMR形态研究确定两个主要络合物,物[Ir(OCHO)(H){κ P,P,的Si-Si(Me)的(C 6 H ^ 4 -2-P我镨2)2 }](2)和物[Ir (H)2 {κP,P,Si-Si(Me)(C 6 H 4 –2-PiPr 2)2 }(DMSO)](3),尽管在当前溶剂系统外不活跃,但可能引起催化循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号