当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and computational study of particle formation kinetics in UF6 hydrolysis
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-07-30 , DOI: 10.1039/d0re00207k Meng-Dawn Cheng 1, 2, 3, 4 , Jason M. Richards 2, 3, 4, 5 , Michael A. Omana 4, 6, 7, 8 , Joshua A. Hubbard 4, 7, 8, 9 , Glenn A. Fugate 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-07-30 , DOI: 10.1039/d0re00207k Meng-Dawn Cheng 1, 2, 3, 4 , Jason M. Richards 2, 3, 4, 5 , Michael A. Omana 4, 6, 7, 8 , Joshua A. Hubbard 4, 7, 8, 9 , Glenn A. Fugate 2, 3, 4, 5
Affiliation
|
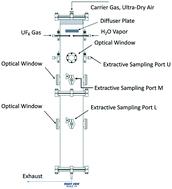
The formation and growth of UO2F2 particles by gas-phase UF6 hydrolysis remains of interest to actinide chemistry researchers. The total number concentration of the UO2F2 aerosol particles that can be produced in the reaction is regulated primarily by the availability of water molecules under our reactor conditions. An increase in water molecule concentration corresponds with a higher amount and larger size of UO2F2 aerosol particles produced. The growth rates of aerosol particles appear to approach a single number in the range of [0.05 ± 0.03–0.08 ± 0.04] (nm s−1), as the molar ratio of water to UF6 decreases below 1. The size of primary particles produced from the UF6 hydrolysis under water-deprived conditions was estimated to be 3.6 ± 0.4 nm. As the molar ratio became greater than 1.7, the size of primary particles increased with increased availability of water molecules. The primary particle model developed in this work predicted a size range for the UO2F2 primary particles similar to that estimated based on the data from gas-phase UF6 hydrolysis experiments. This result suggests that the volume-driven coalescence process assumption used in the derivation of the primary particle model was reasonable. The ability to precisely control the availability of water molecules and reaction time could lead to the production of nearly monodispersed aerosol particles. This finding has significant implications in the engineering and manufacturing of fuel powder materials and possibly the future development and deployment of environmental sampling apparatus.
中文翻译:

UF6水解过程中颗粒形成动力学的实验和计算研究
通过气相UF 6水解形成和生长UO 2 F 2颗粒仍然是act系化学研究人员感兴趣的问题。反应中产生的UO 2 F 2气溶胶颗粒的总浓度主要受反应器条件下水分子的可用性调节。水分子浓度的增加对应于产生的UO 2 F 2气雾剂颗粒的数量更大和尺寸更大。随着水与UF 6的摩尔比,气溶胶颗粒的生长速率似乎在[0.05±0.03-0.08±0.04](nm s -1)范围内接近单个数。降低至低于1。据估计,在缺水条件下,由UF 6水解产生的初级颗粒的大小为3.6±0.4 nm。当摩尔比大于1.7时,初级粒子的尺寸随着水分子的可用性增加而增加。在这项工作中开发的初级粒子模型预测了UO 2 F 2初级粒子的尺寸范围,该尺寸范围类似于基于气相UF 6的数据估算的尺寸范围水解实验。该结果表明,在一次粒子模型的推导中使用的体积驱动的聚结过程假设是合理的。精确控制水分子的可用性和反应时间的能力可能会导致产生几乎单分散的气溶胶颗粒。该发现对燃料粉末材料的工程和制造以及环境采样设备的未来开发和部署具有重要意义。
更新日期:2020-08-25
中文翻译:

UF6水解过程中颗粒形成动力学的实验和计算研究
通过气相UF 6水解形成和生长UO 2 F 2颗粒仍然是act系化学研究人员感兴趣的问题。反应中产生的UO 2 F 2气溶胶颗粒的总浓度主要受反应器条件下水分子的可用性调节。水分子浓度的增加对应于产生的UO 2 F 2气雾剂颗粒的数量更大和尺寸更大。随着水与UF 6的摩尔比,气溶胶颗粒的生长速率似乎在[0.05±0.03-0.08±0.04](nm s -1)范围内接近单个数。降低至低于1。据估计,在缺水条件下,由UF 6水解产生的初级颗粒的大小为3.6±0.4 nm。当摩尔比大于1.7时,初级粒子的尺寸随着水分子的可用性增加而增加。在这项工作中开发的初级粒子模型预测了UO 2 F 2初级粒子的尺寸范围,该尺寸范围类似于基于气相UF 6的数据估算的尺寸范围水解实验。该结果表明,在一次粒子模型的推导中使用的体积驱动的聚结过程假设是合理的。精确控制水分子的可用性和反应时间的能力可能会导致产生几乎单分散的气溶胶颗粒。该发现对燃料粉末材料的工程和制造以及环境采样设备的未来开发和部署具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号