Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-07-31 , DOI: 10.2174/1570180816666191122105313 Shipra Bhati 1 , Vijay Kumar 2 , Simranjeet Singh 3 , Joginder Singh 3
|
Background: Antimicrobial Resistance (AMR) and Tuberculosis (TB) are global concern. According to the WHO fact sheet on tuberculosis, in 2017, 10 million people fell ill with TB, and 1.6 million including 230,000 children died from the disease. There is a critical need of design and development of novel chemotherapeutic agents to combat the emergence and increasing prevalence of resistant pathogens. In the present study, a new series of 1,3,4-oxadiazoles incorporating benzimidazole and pyridine scaffolds in a single molecular framework has been reported.
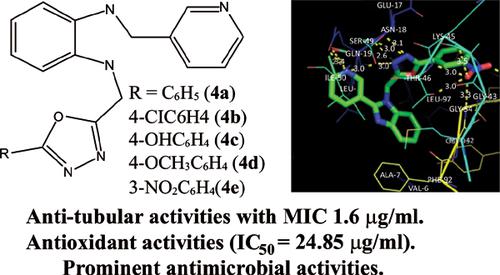
Methods: The structures of the synthesized derivatives (4a to 4e) were assigned by IR, NMR and mass spectral techniques. The hybrid compounds were evaluated for their antimicrobial, antitubercular and antioxidant activities. In addition, docking simulations were performed to study ligand-protein interactions and to determine the probable binding conformations.
Results: Molecule 4a has shown anti-tubular activities with MIC 1.6 μg/ml. As compared to ascorbic acid activities (IC50 = 62.91 μg/ml), molecule 4e exhibited better antioxidant activities (IC50 = 24.85 μg/ml). Also, molecule 4e has shown significant antimicrobial activities.
Conclusion: The synthesized derivatives from 4a to 4e have exhibited various medicinal activities and could be emerged as lead compounds and further explored as potential therapeutic agents.
中文翻译:

2-(吡啶-3-基)-1H苯并[d]咪唑和1,3,4-恶二唑类比衍生物的合成,表征,抗菌,抗结核,抗氧化活性和对接模拟
背景:抗菌素耐药性(AMR)和结核病(TB)是全球关注的问题。根据世卫组织关于结核病的情况说明书,2017年有1000万人患有结核病,其中160万人包括23万儿童死于该病。迫切需要设计和开发新型化学治疗剂来抵抗耐药病原体的出现和流行。在本研究中,已经报道了在单个分子框架中结合了苯并咪唑和吡啶骨架的一系列新的1,3,4-恶二唑。
方法:通过红外,核磁共振和质谱技术确定合成衍生物(4a至4e)的结构。评价杂合化合物的抗微生物,抗结核和抗氧化活性。另外,进行对接模拟以研究配体-蛋白质相互作用并确定可能的结合构象。
结果:分子4a具有MIC 1.6μg/ ml的抗微管活性。与抗坏血酸活性(IC50 = 62.91μg/ ml)相比,分子4e表现出更好的抗氧化活性(IC50 = 24.85μg/ ml)。而且,分子4e显示出显着的抗微生物活性。
结论:合成的4a至4e衍生物具有多种药物活性,可以作为先导化合物出现,并可以进一步开发为潜在的治疗药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号