当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of (-)-Melodinine K: A Case Study of Efficiency in Natural Product Synthesis.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-07-29 , DOI: 10.1021/acs.jnatprod.0c00310 Manish Walia 1 , Christiana N Teijaro 1 , Alex Gardner 1 , Thi Tran 1 , Jinfeng Kang 1 , Senzhi Zhao 1 , Sarah E O'Connor 2 , Vincent Courdavault 3 , Rodrigo B Andrade 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-07-29 , DOI: 10.1021/acs.jnatprod.0c00310 Manish Walia 1 , Christiana N Teijaro 1 , Alex Gardner 1 , Thi Tran 1 , Jinfeng Kang 1 , Senzhi Zhao 1 , Sarah E O'Connor 2 , Vincent Courdavault 3 , Rodrigo B Andrade 1
Affiliation
|
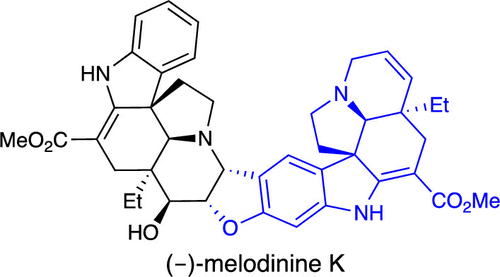
Efficiency is a key organizing principle in modern natural product synthesis. Practical criteria include time, cost, and effort expended to synthesize the target, which tracks with step-count and scale. The execution of a natural product synthesis, that is, the sum and identity of each reaction employed therein, falls along a continuum of chemical (abiotic) synthesis on one extreme, followed by the hybrid chemoenzymatic approach, and ultimately biological (biosynthesis) on the other, acknowledging the first synthesis belongs to Nature. Starting materials also span a continuum of structural complexity approaching the target with constituent elements on one extreme, followed by petroleum-derived and “chiral pool” building blocks, and complex natural products (i.e., semisynthesis) on the other. Herein, we detail our approach toward realizing the first synthesis of (−)-melodinine K, a complex bis-indole alkaloid. The total syntheses of monomers (−)-tabersonine and (−)-16-methoxytabersonine employing our domino Michael/Mannich annulation is described. Isolation of (−)-tabersonine from Voacanga africana and strategic biotransformation with tabersonine 16-hydroxylase for site-specific C–H oxidation enabled a scalable route. The Polonovski–Potier reaction was employed in biomimetic fragment coupling. Subsequent manipulations delivered the target. We conclude with a discussion of efficiency in natural products synthesis and how chemical and biological technologies define the synthetic frontier.
中文翻译:

(-)-Melodinine K 的合成:天然产物合成效率的案例研究。
效率是现代天然产物合成的关键组织原则。实际标准包括时间、成本和合成目标所花费的精力,这些目标通过步数和规模进行跟踪。天然产物合成的执行,即其中使用的每个反应的总和和同一性,在一个极端上是化学(非生物)合成的连续统,然后是混合化学酶法,最后是生物(生物合成)其他,承认第一次综合属于自然。起始材料还跨越了结构复杂性的连续统一体,在一个极端接近目标的组成元素,其次是石油衍生和“手性池”构建块,以及复杂的天然产物(即半合成)。在此,我们详细介绍了实现 (-)-melodinine K(一种复杂的双吲哚生物碱)首次合成的方法。描述了使用我们的多米诺迈克尔/曼尼希环化的单体 (-)-tabersonine 和 (-)-16-甲氧基tabersonine 的全合成。从Voacanga Africana 中分离 (-)-tabersonine使用 tabersonine 16-羟化酶进行位点特异性 C-H 氧化的战略性生物转化实现了可扩展的路线。Polonovski-Potier 反应用于仿生片段偶联。随后的操作传递了目标。我们最后讨论了天然产物合成的效率以及化学和生物技术如何定义合成前沿。
更新日期:2020-08-28
中文翻译:

(-)-Melodinine K 的合成:天然产物合成效率的案例研究。
效率是现代天然产物合成的关键组织原则。实际标准包括时间、成本和合成目标所花费的精力,这些目标通过步数和规模进行跟踪。天然产物合成的执行,即其中使用的每个反应的总和和同一性,在一个极端上是化学(非生物)合成的连续统,然后是混合化学酶法,最后是生物(生物合成)其他,承认第一次综合属于自然。起始材料还跨越了结构复杂性的连续统一体,在一个极端接近目标的组成元素,其次是石油衍生和“手性池”构建块,以及复杂的天然产物(即半合成)。在此,我们详细介绍了实现 (-)-melodinine K(一种复杂的双吲哚生物碱)首次合成的方法。描述了使用我们的多米诺迈克尔/曼尼希环化的单体 (-)-tabersonine 和 (-)-16-甲氧基tabersonine 的全合成。从Voacanga Africana 中分离 (-)-tabersonine使用 tabersonine 16-羟化酶进行位点特异性 C-H 氧化的战略性生物转化实现了可扩展的路线。Polonovski-Potier 反应用于仿生片段偶联。随后的操作传递了目标。我们最后讨论了天然产物合成的效率以及化学和生物技术如何定义合成前沿。











































 京公网安备 11010802027423号
京公网安备 11010802027423号