当前位置:
X-MOL 学术
›
Mater. Corros.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The anoxic corrosion behaviour of copper in the presence of chloride and sulphide
Materials and Corrosion ( IF 1.6 ) Pub Date : 2020-07-29 , DOI: 10.1002/maco.202011783 Nicholas A. Senior 1 , Taylor Martino 1 , Jeff Binns 2 , Peter Keech 2
Materials and Corrosion ( IF 1.6 ) Pub Date : 2020-07-29 , DOI: 10.1002/maco.202011783 Nicholas A. Senior 1 , Taylor Martino 1 , Jeff Binns 2 , Peter Keech 2
Affiliation
|
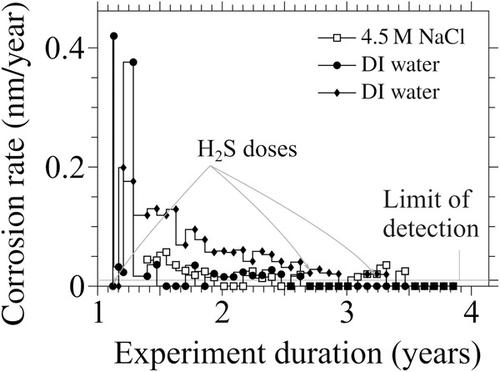
The Canadian used fuel container for the long‐term containment of spent nuclear fuel in a deep geological repository comprises a 3‐mm copper corrosion barrier applied directly to a strong carbon steel container. Although a final site for the Canadian deep geological repository has not yet been chosen, the site selection process has narrowed down to two candidate locations with unique groundwater chemistry, particularly with respect to salinity. Therefore, to ensure the long‐term integrity of the used fuel container, the effect of a range of groundwater chemistries on copper corrosion must be understood. The primary variables of interest are the influence of chloride concentration and the consequences of the interaction of hydrogen sulphide, naturally present in the groundwaters in very low concentrations, with the copper cladding. An additional aspect that has been investigated is the behaviour of copper in pure water. The anoxic corrosion of copper can be inferred by the very slow release of hydrogen gas. In this paper, new data are presented, which demonstrate an extremely sensitive approach to quantify the very limited hydrogen release at 75°C. Initially, corrosion rates under conditions approximating an anticipated Canadian deep geological repository were significantly less than 0.5 nm/year and they invariably declined over the course of several months/years. The presence of chloride or hydrogen sulphide was found, under specific conditions, to stimulate short‐term corrosion behaviour, which was consistent with anion‐assisted surface rearrangement, not bulk corrosion.
中文翻译:

氯化物和硫化物存在下铜的缺氧腐蚀行为
加拿大的用于长期将乏核燃料容纳在深层地质处置库中的燃料容器包括3毫米的铜腐蚀防护层,可直接应用于坚固的碳钢容器。尽管尚未选择加拿大深层地质处置库的最终站点,但站点选择过程已缩小到两个具有独特地下水化学特征的候选位置,特别是在盐度方面。因此,为了确保用过的燃料容器的长期完整性,必须了解一系列地下水化学物质对铜腐蚀的影响。感兴趣的主要变量是氯化物浓度的影响以及地下水中天然存在的硫化氢与覆铜层相互作用的后果。已研究的另一个方面是纯水中铜的行为。氢气的缓慢释放可以推断出铜的缺氧腐蚀。在本文中,提供了新的数据,这些数据展示了一种非常灵敏的方法来量化在75°C时非常有限的氢气释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。氢气的缓慢释放可以推断出铜的缺氧腐蚀。在本文中,提供了新的数据,这些数据展示了一种非常灵敏的方法来量化在75°C时非常有限的氢气释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。氢气的缓慢释放可以推断出铜的缺氧腐蚀。在本文中,提供了新的数据,这些数据展示了一种非常灵敏的方法来量化在75°C时非常有限的氢气释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。该方法展示了一种非常灵敏的方法来定量在75°C时非常有限的氢释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。该方法展示了一种非常灵敏的方法来定量在75°C时非常有限的氢释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。
更新日期:2020-07-29
中文翻译:

氯化物和硫化物存在下铜的缺氧腐蚀行为
加拿大的用于长期将乏核燃料容纳在深层地质处置库中的燃料容器包括3毫米的铜腐蚀防护层,可直接应用于坚固的碳钢容器。尽管尚未选择加拿大深层地质处置库的最终站点,但站点选择过程已缩小到两个具有独特地下水化学特征的候选位置,特别是在盐度方面。因此,为了确保用过的燃料容器的长期完整性,必须了解一系列地下水化学物质对铜腐蚀的影响。感兴趣的主要变量是氯化物浓度的影响以及地下水中天然存在的硫化氢与覆铜层相互作用的后果。已研究的另一个方面是纯水中铜的行为。氢气的缓慢释放可以推断出铜的缺氧腐蚀。在本文中,提供了新的数据,这些数据展示了一种非常灵敏的方法来量化在75°C时非常有限的氢气释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。氢气的缓慢释放可以推断出铜的缺氧腐蚀。在本文中,提供了新的数据,这些数据展示了一种非常灵敏的方法来量化在75°C时非常有限的氢气释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。氢气的缓慢释放可以推断出铜的缺氧腐蚀。在本文中,提供了新的数据,这些数据展示了一种非常灵敏的方法来量化在75°C时非常有限的氢气释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。该方法展示了一种非常灵敏的方法来定量在75°C时非常有限的氢释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。该方法展示了一种非常灵敏的方法来定量在75°C时非常有限的氢释放。最初,在接近预期的加拿大深层地质处置库的条件下,腐蚀速率显着低于0.5 nm /年,并且在数月/年的过程中始终会下降。在特定条件下,发现氯化物或硫化氢的存在会刺激短期腐蚀行为,这与阴离子辅助的表面重排一致,而不是整体腐蚀。











































 京公网安备 11010802027423号
京公网安备 11010802027423号