Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Penetrable Nanoplatform for “Cold” Tumor Immune Microenvironment Reeducation
Advanced Science ( IF 14.3 ) Pub Date : 2020-07-29 , DOI: 10.1002/advs.202000411 Qinjun Chen 1 , Yongqing He 1 , Yu Wang 1 , Chao Li 1 , Yujie Zhang 1 , Qin Guo 1 , Yiwen Zhang 1 , Yongchao Chu 1 , Peixin Liu 1 , Hongyi Chen 1 , Zheng Zhou 1 , Wenxi Zhou 1 , Zhenhao Zhao 1 , Xiaomin Li 2 , Tao Sun 1 , Chen Jiang 1
Advanced Science ( IF 14.3 ) Pub Date : 2020-07-29 , DOI: 10.1002/advs.202000411 Qinjun Chen 1 , Yongqing He 1 , Yu Wang 1 , Chao Li 1 , Yujie Zhang 1 , Qin Guo 1 , Yiwen Zhang 1 , Yongchao Chu 1 , Peixin Liu 1 , Hongyi Chen 1 , Zheng Zhou 1 , Wenxi Zhou 1 , Zhenhao Zhao 1 , Xiaomin Li 2 , Tao Sun 1 , Chen Jiang 1
Affiliation
|
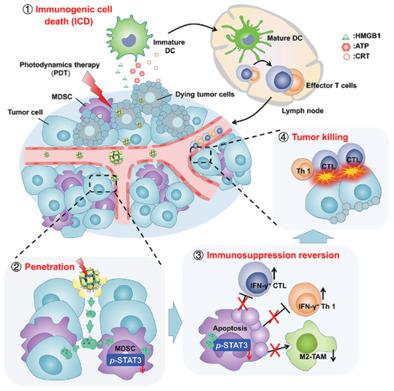
Lack of tumor‐infiltration lymphocytes (TILs) and resistances by overexpressed immunosuppressive cells (principally, myeloid‐derived suppressor cells (MDSCs)) in tumor milieu are two major challenges hindering the effectiveness of immunotherapy for “immune‐cold” tumors. In addition, the natural physical barrier existing in solid cancer also limits deeper delivery of drugs. Here, a tumor‐targeting and light‐responsive‐penetrable nanoplatform (Apt/PDGs^s@pMOF) is developed to elicit intratumoral infiltration of cytotoxic T cells (CTLs) and reeducate immunosuppressive microenvironment simultaneously. In particular, porphyrinic metal–organic framework (pMOF)–based photodynamic therapy (PDT) induces tumor immunogenic cell death (ICD) to promote CTLs intratumoral infiltration and hot “immune‐cold” tumor. Upon being triggered by PDT, the nearly 10 nm adsorbed drug‐loaded dendrimer de‐shields from the nanoplatform and spreads into the deeper tumor, eliminating MDSCs and reversing immunosuppression, eventually reinforcing immune response. Meanwhile, the designed nanoplatform also has a systemic MDSC inhibition effect and moderate improvement of overall antitumor immune responses, resulting in effective suppression of distal tumors within less significant immune‐related adverse effects (irAEs) induced.
中文翻译:

用于“冷”肿瘤免疫微环境再教育的可穿透纳米平台
肿瘤环境中肿瘤浸润淋巴细胞(TIL)的缺乏和过度表达的免疫抑制细胞(主要是骨髓源性抑制细胞(MDSC))的抵抗是阻碍“免疫冷”肿瘤免疫治疗有效性的两个主要挑战。此外,实体瘤中存在的天然物理屏障也限制了药物的更深层次输送。在这里,开发了一种肿瘤靶向和光响应可穿透的纳米平台(Apt/PDGs ^ s@pMOF)来引发细胞毒性T细胞(CTL)的瘤内浸润并同时重新教育免疫抑制微环境。特别是,基于卟啉金属有机框架(pMOF)的光动力疗法(PDT)诱导肿瘤免疫原性细胞死亡(ICD),从而促进CTL瘤内浸润和热“免疫冷”肿瘤。在 PDT 触发后,近 10 nm 吸附的载药树枝状大分子脱离纳米平台并扩散到更深的肿瘤中,消除 MDSC 并逆转免疫抑制,最终增强免疫反应。同时,设计的纳米平台还具有系统性MDSC抑制作用,并适度改善整体抗肿瘤免疫反应,从而在引起不太显着的免疫相关不良反应(irAE)的情况下有效抑制远端肿瘤。
更新日期:2020-09-10
中文翻译:

用于“冷”肿瘤免疫微环境再教育的可穿透纳米平台
肿瘤环境中肿瘤浸润淋巴细胞(TIL)的缺乏和过度表达的免疫抑制细胞(主要是骨髓源性抑制细胞(MDSC))的抵抗是阻碍“免疫冷”肿瘤免疫治疗有效性的两个主要挑战。此外,实体瘤中存在的天然物理屏障也限制了药物的更深层次输送。在这里,开发了一种肿瘤靶向和光响应可穿透的纳米平台(Apt/PDGs ^ s@pMOF)来引发细胞毒性T细胞(CTL)的瘤内浸润并同时重新教育免疫抑制微环境。特别是,基于卟啉金属有机框架(pMOF)的光动力疗法(PDT)诱导肿瘤免疫原性细胞死亡(ICD),从而促进CTL瘤内浸润和热“免疫冷”肿瘤。在 PDT 触发后,近 10 nm 吸附的载药树枝状大分子脱离纳米平台并扩散到更深的肿瘤中,消除 MDSC 并逆转免疫抑制,最终增强免疫反应。同时,设计的纳米平台还具有系统性MDSC抑制作用,并适度改善整体抗肿瘤免疫反应,从而在引起不太显着的免疫相关不良反应(irAE)的情况下有效抑制远端肿瘤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号